Innovative Technology for Parathyroid Gland Identification
Though early in its evolution, the adoption of NIRAF in thyroid and parathyroid surgery seems likely to grow as more evidence regarding its advantages becomes available.
Julia E. Noel, MD, Amanda L. Silver Karcioglu, MD, Gregory W. Randolph, MD, and Michael C. Singer, MD
Since the development of modern thyroid surgery techniques in the late 19th century, hypoparathyroidism has threatened the safety and health of patients in the post-thyroidectomy period. Even the preeminent surgeon Theodor Billroth struggled with this complication, with postoperative tetany leading to the death of a number of his patients.1
Although the rate of permanent hypoparathyroidism is often quoted as occurring in less than 1% of thyroidectomies, large registry studies have now revealed that the true incidence is likely 10% or higher.2 Furthermore, the long-term consequences of this complication are now beginning to be more fully understood. Permanent hypoparathyroidism, even with well-maintained calcium levels, has repercussions for patients’ renal, cardiac, neurologic, and bone health; significantly impairs quality of life; and potentially even impacts life expectancy.3
Historically, avoiding hypoparathyroidism by recognizing, preserving, and then assessing the viability of the parathyroid glands (PGs) relied fully on the skill and judgment of the surgeon. Surgeons have been taught to employ a “capsular dissection” technique in an effort to leave the PGs in-situ, with an intact terminal blood supply. However, given the rates of temporary and permanent hypoparathyroidism, it is clear that enhanced approaches to PG preservation would be beneficial.
New technologies that serve as intraoperative tools to aid in PG recognition and preservation were the focus of a Panel Presentation at the AAO-HNSF 2022 Annual Meeting & OTO Experience. These technologies, which use near infrared imaging (NIRI) with endogenous PG autofluorescence (NIRAF) or with the aid of indocyanine green (ICG), are early in their development. A Panel Presentation on this topic will again be presented at the upcoming 2023 Annual Meeting in Nashville, Tennessee, September 30 – October 4.
PG autofluorescence (NIRAF) has recently garnered significant research interest. Autofluorescence is distinct optically from other fluorescence techniques that rely on an injectable agent (such as ICG) to emit a stimulated wavelength of light. In NIRAF, no adjuvant agent is used to induce fluorescence. Rather an intrinsic fluorophore responds to the light excitation. PGs have an endogenous fluorophore, as yet identified, that emits NIR light at a peak emission wavelength of 820nm when illuminated with light at 785nm. Importantly, NIRAF is not related to perfusion, and the autofluorescence quality maintains itself even if the tissue is devascularized, frozen, or fixed in formalin. Consequently, NIRAF is useful for PG identification but not to assess vascular viability. Currently, use of ICG injection is still needed if assessment of perfusion is desired.
Critically, PGs demonstrate significantly higher degrees of autofluorescence compared to all other adjacent structures in the neck, including the thyroid, lymph nodes, fat, and thymus.4
Different technologies are now available to exploit the conspicuous autofluorescent nature of PGs. Camera-based systems employ a light source and camera set to the appropriate wavelengths and demonstrate the images on a monitor in real time. Frequently, the PGs differentiate themselves by their intense autofluorescence compared to other tissues.
In addition to the camera-based systems, a probe-based option is available. This device houses both the stimulator and receiver in a low-profile, handheld probe. When the probe is applied to tissue, the accompanying monitor shows its absolute degree of autofluorescence and a ratio compared with the measured baseline autofluorescence of the thyroid. A ratio above a certain amount indicates that the contacted tissue is likely parathyroid.
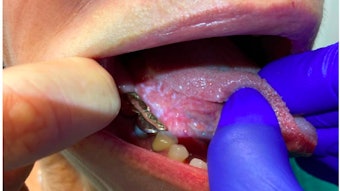
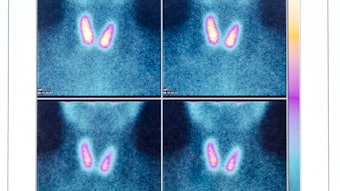
 A near infrared camera system demonstrates the relatively bright autofluorescence of a parathyroid gland against the thyroid lobe.
A near infrared camera system demonstrates the relatively bright autofluorescence of a parathyroid gland against the thyroid lobe.
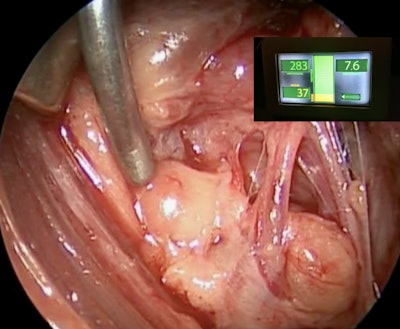 The probe-based system provides a quantitative measure of the degree of autofluorescence of a parathyroid gland (being stimulated) compared to the thyroid. A high detection ratio strongly indicates that the tested tissue is parathyroid.
The probe-based system provides a quantitative measure of the degree of autofluorescence of a parathyroid gland (being stimulated) compared to the thyroid. A high detection ratio strongly indicates that the tested tissue is parathyroid.
Studies and Investigations
Results from early studies examining the value of these technologies in reducing rates of hypoparathyroidism have been mixed but promising. There is clear evidence that the number of PGs removed inadvertently during surgery when employing NIRAF is reduced significantly.5 Furthermore, rates of temporary hypoparathyroidism have been shown to be similar positively impacted.6 To date, the outcomes with permanent hypoparathyroidism are less convincing, though several studies have shown a reduction in this complication.7 Larger studies, including surgeons with lower thyroidectomy volumes, need to be conducted to more fully establish the complication-lowering ability of NIRAF.
A number of additional, crucial questions are actively being investigated. Most importantly is the optimal manner in which these technologies should be incorporated into the surgical workflow. For example, at which point(s) during thyroidectomy should NIRAF be employed. There is universal agreement that NIRAF can be used to exam the surgical specimen after it has been removed to reveal any inadvertently excised PGs. But for earlier stages of the surgery, there is less agreement. Researchers are trying to establish clear protocols for NIRAF similar to the pathways that have been described for the optimal utilization of neuromonitoring during thyroid surgery.8,9 A recent review from the Endocrine Surgery Section of the American Head and Neck Society provides an up-to-date summary of the current state of NIRAF and associated recommendations.8 As NIRAF-related technologies and techniques improve, these protocols should become more refined and precise.
To date, most of the research assessing these technologies has examined their utility in thyroid surgery in an effort to reduce postoperative hypoparathyroidism. But their ability to help identify PGs clearly lends itself to their incorporation into parathyroid surgery as well. In some parathyroidectomies, identifying the PGs can be challenging and failure to find them can lead to surgical failure. Although it seems that NIRAF will in the future play a significant role in parathyroid surgery, further research is required to understand how it should be applied to provide genuine and cost-effective benefits.
Though early in its evolution, the adoption of NIRAF in thyroid and parathyroid surgery seems likely to grow as more evidence regarding its advantages becomes available. As has happened with neuromonitoring, this technology could become an essential element of these endocrine surgeries.
References
- Sakorafas GH. Historical evolution of thyroid surgery: from the ancient times to the dawn of the 21st century. World J Surg. 2010 Aug;34(8):1793-804. doi: 10.1007/s00268-010-0580-7.
- Annebäck M, Hedberg J, Almquist M, Stålberg P, Norlén O. Risk of permanent hypoparathyroidism after total thyroidectomy for benign disease: A nationwide population-based cohort study from Sweden. Ann Surg. 2021 Dec 1;274(6):e1202-e1208. doi: 10.1097/SLA.0000000000003800.
- Almquist M, Ivarsson K, Nordenström E, Bergenfelz A. Mortality in patients with permanent hypoparathyroidism after total thyroidectomy. Br J Surg. 2018 Sep;105(10):1313-1318. doi: 10.1002/bjs.10843. Epub 2018 Apr 17.
- McWade MA, Sanders ME, Broome JT, Solórzano CC, Mahadevan-Jansen A. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery. 2016 Jan;159(1):193-202. doi: 10.1016/j.surg.2015.06.047. Epub 2015 Oct 9..
- Benmiloud F, Godiris-Petit G, Gras R, Gillot JC, Turrin N, Penaranda G, Noullet S, Chéreau N, Gaudart J, Chiche L, Rebaudet S. Association of autofluorescence-based detection of the parathyroid glands during total thyroidectomy with postoperative hypocalcemia risk: Results of the PARAFLUO multicenter randomized clinical trial. JAMA Surg. 2020 Feb 1;155(2):106-112. doi: 10.1001/jamasurg.2019.4613.
- Dip F, Falco J, Verna S, Prunello M, Loccisano M, Quadri P, White K, Rosenthal R. Randomized controlled trial comparing white light with near-infrared autofluorescence for parathyroid gland identification during total thyroidectomy. J Am Coll Surg. 2019 May;228(5):744-751. doi: 10.1016/j.jamcollsurg.2018.12.044. Epub 2019 Jan 31.
- Weng YJ, Jiang J, Min L, Ai Q, Chen DB, Chen WC, Huang ZH. Intraoperative near-infrared autofluorescence imaging for hypocalcemia risk reduction after total thyroidectomy: Evidence from a meta-analysis. Head Neck. 2021 Aug;43(8):2523-2533. doi: 10.1002/hed.26733. Epub 2021 May 5.
- Silver Karcioglu AL, Triponez F, Solórzano CC, Iwata AJ, Abdelhamid Ahmed AH, Almquist M, Angelos P, Benmiloud F, Berber E, Bergenfelz A, Cha J, Colaianni CA, Davies L, Duh QY, Hartl D, Kandil E, Kim WW, Kopp PA, Liddy W, Mahadevan-Jansen A, Lee KD, Mannstadt M, McMullen CP, Shonka DC Jr, Shin JJ, Singer MC, Slough CM, Stack BC Jr, Tearney G, Thomas G, Tolley N, Vidal-Fortuny J, Randolph GW. Emerging imaging technologies for parathyroid gland identification and vascular assessment in thyroid surgery: A review from the American Head and Neck Society Endocrine Surgery Section. JAMA Otolaryngol Head Neck Surg. 2023 Mar 1;149(3):253-260. doi: 10.1001/jamaoto.2022.4421.
- Randolph GW, Dralle H; International ntraoperative Monitoring Study Group; Abdullah H, Barczynski M, Bellantone R, Brauckhoff M, Carnaille B, Cherenko S, Chiang FY, Dionigi G, Finck C, Hartl D, Kamani D, Lorenz K, Miccolli P, Mihai R, Miyauchi A, Orloff L, Perrier N, Poveda MD, Romanchishen A, Serpell J, Sitges-Serra A, Sloan T, Van Slycke S, Snyder S, Takami H, Volpi E, Woodson G. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011 Jan;121 Suppl 1:S1-16. doi: 10.1002/lary.21119.