The Joint Committee on Infant Hearing
The principles and guidelines of early hearing detection and intervention programs.
Ursula M. Findlen, PhD, Craig A. Buchman, MD, Stephanie Moody-Antonio, MD, Oliver F. Adunka, MD, MBA
In recognition of the need to identify hearing impairment as early in life as possible, auditory screening programs have been implemented across all 50 states. However, it was not until the 1960s that the importance of early hearing screening was recognized. As a result, in 1969, representatives from the American Academy of Ophthalmology and Otolaryngology, the American Academy of Pediatrics, and the American Speech & Hearing Association formed the Joint Committee on Infant Hearing (JCIH). The first position statement, which was published in 1971, recognized the importance of infant hearing screening.1 However, this first position statement cautioned that the results of mass screening programs were inconsistent and often misleading, ultimately recommending increased research efforts without routine screening of newborn infants.
Through technical and clinical advancements, subsequent JCIH statements were able to provide more specific screening as well as diagnostic recommendations. Specifically, risk factors for hearing loss and proper clinical protocols were more accurately delineated. Also, electrophysiologic testing methods were first recommended to screen for newborn infant hearing loss. However, it was not until the 1994 statement that the JCIH endorsed universal detection of hearing loss in newborns and infants.2 In addition, the 1-3-6 principle was introduced in the statement from the year 2000, which describes the key components of early hearing detection and intervention programs (EHDI). The EHDI timeline recommends screening by one month of age, diagnostic audiological and medical evaluation by three months of age, and initiation of state-provided early intervention developmental services (e.g., developmental home programming, speech/language therapy services, etc.) by six months of age. This approach to EHDI is still used today.3
Current State of the JCIH
Over the years, and with newborn infant hearing screening implemented in all 50 states and multiple territories, the JCIH statement has evolved into a complex document. This is also a result of the expansion of the JCIH, now including many stakeholder organizations that represent diverse professional, educational, and experiential perspectives. (Visit www.jcih.org for a list of member organization). In addition, technological advancements and novel clinical data have allowed specific screening and follow-up recommendations to evolve. The most recent statement released in 2019 thoroughly outlines the evidence base for 1-3-6 and makes specific recommendations for the effective identification and management of congenital hearing loss via family-centered care.4
Some of the more substantive changes in this latest iteration include highlighting the effectiveness of screening with otoacoustic emissions (OAE) in the well-baby population to identify hearing loss due to transient middle ear pathology, updating the follow-up recommendations for infants who pass screening at birth but are at high risk for late-onset or progressive hearing loss, and recommending that programs meeting 1-3-6 standards should consider striving for a 1-2-3 process, that is, screening by one month, diagnosis by two months, and implementation of early intervention by three months.
Screening techniques can include either OAE testing or automated auditory brainstem response (AABR) testing or a combination of both techniques, depending upon state regulations or program recommendations. Both techniques have advantages and disadvantages. OAEs are impacted by the outer and middle ear status of the infant and therefore can yield a non-pass result due to transient or conductive issues. AABR uses template matching for wave V identification and can yield a pass result for infants who have mild hearing loss, regardless of type (conductive, mixed, or sensorineural). Both techniques are subject to patient and environmental noise that can yield erroneous results. The current 2019 JCIH statement endorses the use of OAEs for both initial and subsequent screening of well babies given reduced costs and very low risk of auditory neuropathy. OAEs are also more sensitive in identifying mild hearing loss missed by AABR, hearing loss that can still be developmentally significant. AABR continues to be the recommended technique for babies in the neonatal intensive care unit (NICU), given higher risk for elevated hearing thresholds and auditory neuropathy spectrum disorder.
Perhaps the most substantive change in the 2019 JCIH statement was the recommendation for a more aggressive follow-up cadence for infants with risk factors (Table 1). Previously, follow-up hearing monitoring was recommended between 24 and 30 months of age to evaluate whether the infant had late-onset or progressive hearing loss. New recommendations range from no later than one month for infants with Zika, three months for infants with congenital cytomegalovirus (CMV) and/or history of extracorporeal membrane oxygenation, and nine months for all other risk factors. It was emphasized that diagnostic assessment is critical to identify hearing loss, regardless of the status of initial, early screening result.
Finally, an aspirational recommendation was made for programs meeting the 1-3-6 timeline to consider evolving to an even shorter 1-2-3 timeline. Short- and long-term language outcomes are known to be better when enrollment into a state-provided early intervention program is initiated by six months of age.5-7 Emerging data support that enrollment in early intervention by three months improves global language8; however, additional studies are needed to replicate these findings in larger and more diverse populations.
Table 1. Risk factors for early childhood hearing loss.
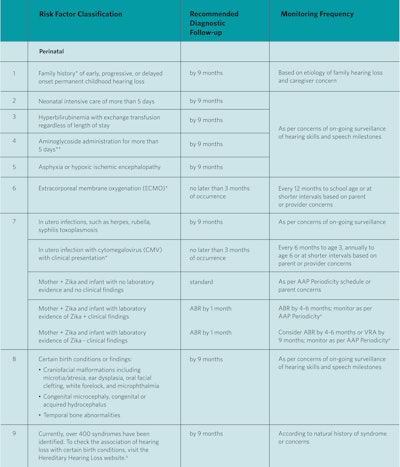 Note: Adapted from Table 1 in the 2019 JCIH Position Statement. AAP = American Academy of Pediatrics; ABR = auditory brainstem response; VRA = visual reinforcement audiometry.
Note: Adapted from Table 1 in the 2019 JCIH Position Statement. AAP = American Academy of Pediatrics; ABR = auditory brainstem response; VRA = visual reinforcement audiometry.
* Infants at increased risk of delayed onset or progressive hearing loss.
**Infants with toxic levels or with a known genetic susceptibility remain at risk.
a Adebanjo T, Godfred-Cato S, Viens L, et al. Update: interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection—United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017;66(41):1089–1099.
b Hereditary Hearing Loss homepage. http://hereditaryhearingloss.org/.
Current State of EHDI
Although the 1-3-6 template has been the goal of EHDI for over 20 years, there is no universal federal mandate or funding, and each state or territory EHDI program works under its unique laws, policies, and regulations; subsequently, each EHDI program’s ability to meet these goals varies considerably.9 State EHDI programs typically report statistics to the Center for Disease Control and Prevention annually but with a two-year lag (e.g., 2020 data were reported in 2022). Compliance with newborn hearing screening has increased steadily, achieving an overall rate of 97.9% of newborns screened across 56 states and territories in 2020.10 Despite success at the screening stage, compliance with diagnostic evaluation and initiation of early intervention lags behind (regardless of timing), overall reported to be 60.0% and 61.4%, respectively (Figure 1). There are likely several issues contributing to the poor compliance with diagnostic evaluation and referral to early intervention. Within the policies and regulations guiding each EHDI program, there are significant variations in the standards for screening and requirements for reporting to the state’s department of health. Most notably, even though screening by one month is mandated by state or territory law, the timing for diagnosis and early intervention enrollment is not. This has prompted some to call for a universal standard to improve programs and outcomes for infants with congenital hearing loss regardless of where they are born.11,12 Moreover, most state laws mandating universal newborn hearing screening are unfunded mandates, which puts them at continual risk of funding problems.
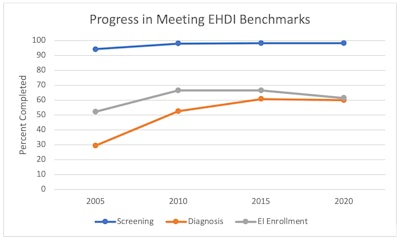 Figure 1: Percentage of completed screening, diagnosis, and enrollment into early intervention from 2005 to 2020. Adapted from annual summary of national CDC EHDI data. Rates reflect percent completed regardless of timing (i.e., screened, diagnosed, and enrolled into early intervention at any time during the reporting period).
Figure 1: Percentage of completed screening, diagnosis, and enrollment into early intervention from 2005 to 2020. Adapted from annual summary of national CDC EHDI data. Rates reflect percent completed regardless of timing (i.e., screened, diagnosed, and enrolled into early intervention at any time during the reporting period).
The Centers for Disease Control and Prevention (CDC) supports funding for data collection, management, and research while the Health Resources and Services Administration (HRSA) provides funding to EHDI programs to support continuous improvement through grants. These grants help to identify effective strategies to improve screening, reduce loss to follow-up rates, provide education for healthcare providers, and improve family engagement and support. HRSA and state funding do not contribute to birthing facilities administering newborn screening programs.
Funding for EHDI through the CDC, HRSA, and the National Institute on Deafness and Other Communications Disorders is under continual risk and requires reauthorization. In December 2022 the Early Hearing Detection and Intervention Act of 2022 was passed by Congress and signed by President Biden reauthorizing through FY 2027 activities that support screening and early intervention for hearing loss in newborns, infants, and children. Although HRSA funding can contribute to quality improvement measures and positively impact outcomes, diagnostic assessment of infants is not usually funded by HRSA; rather, these costs are charged to the family’s insurance from the provider rendering those services similar to screening charges at the birth hospital. Ultimately, although universal screening is mandated and diagnostic follow-up is recommended, families and insurers are responsible for charges associated with services rendered.
In the absence of a federally mandated universal standard, the medical home plays a critical role in the continued progress toward effective diagnostic follow-up and early intervention. Primary care physicians serve as the referral source for families to be seen by audiologists for diagnosis, as well as entrance into a family’s state early intervention program. Unfortunately, evidence has shown across multiple decades that lack of provider knowledge about EHDI and the need for follow-up has served as a barrier to families effectively carrying out recommended follow-up.13-15 These barriers are even more pronounced for infants who pass the newborn screening but present with risk factors that require monitoring and follow-up.16-18
Consequently, otolaryngologists play a crucial role in assuring appropriate referral to our audiology, speech pathology, and early intervention partners when providing care to infants and young children. Incorporating a history about newborn hearing screening and follow-up into the intake for all pediatric patients serves to identify risk for hearing loss and the need for appropriate referrals. Additionally, the expertise that otolaryngologists have in ear and hearing care can provide helpful insight to local- and state-level quality improvement initiatives focused on improvement of diagnosis and early intervention. Otolaryngologists, the American Academy of Otolaryngology–Head and Neck Surgery, and our state otolaryngology societies play a vital role in advocacy for laws, regulations, and funding that are necessary to support the EHDI programs. The National Technical Resource Center managed by the National Center for Hearing Assessment and Management (NCHAM) serves as a central resource center to support EHDI programs. More information about EHDI, state-specific resources, and initiatives can be found at www.infanthearing.org.
References
- Joint Committee on Infant Hearing. Joint statement on neonatal screening for hearing impairment. 1971. http://www.jcih.org/JCIH1971.pdf
- Joint Committee on Infant Hearing. 1994 position statement. 1994. http://www.jcih.org/JCIH1994.pdf
- Joint Committee on Infant Hearing. Year 2000 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2000;106(4):798-817.
- Joint Committee on Infant Hearing. Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs. J Early Hear Detect Interv. 2019;4(2):1-44. doi:10.15142/fptk-b748
- Yoshinaga-Itano C, Sedey AL, Wiggin M, Chung W. Early hearing detection and vocabulary of children with hearing loss. Pediatrics. 2017;140(2). doi:10.1542/peds.2016-2964
- Meinzen-Derr J, Wiley S, Grove W, et al. Kindergarten readiness in children who are deaf or hard of hearing who received early intervention. Published online 2020. Accessed August 26, 2021. www.aappublications.org/news
- Grey B, Deutchki EK, Lund EA, Werfel KL. Impact of meeting early hearing detection and intervention benchmarks on spoken language. Published online 2021. doi:10.1177/10538151211025210
- Walker E, Ward C, Oleson J, et al. Language growth in children with mild to severe hearing loss who received early intervention by 3 months or 6 months of age. J Early Hear Detect Interv. 2022;7(1). Accessed May 12, 2022. https://digitalcommons.usu.edu/jehdi/vol7/iss1/2
- Subbiah K, Mason CA, Gaffney M, Grosse SD. Progress in documented early identification and intervention for deaf and hard of hearing infants: CDC’s hearing screening and follow-up. 2018;3(2):1-7.
- Centers for Disease Control. Annual data: Early Hearing Detection and Intervention (EHDI) program. https://www.cdc.gov/ncbddd/hearingloss/ehdi-data.html
- Smith B, Zhang J, Pham GN, et al. Effects of socioeconomic status on children with hearing loss. Int J Pediatr Otorhinolaryngol. 2019;116(PG-114-117):114-117.
- Kingsbury S, Khvalabov N, Stirn J, et al. Barriers to equity in pediatric hearing health care: a review of the evidence. Perspect ASHA Spec Interest Groups. 2022;7(4): 1060-1071. doi:10.1044/2021_persp-21-00188
- Brown NC, James K, Liu J, Hatcher PA, Li Y. Newborn hearing screening. An assessment of knowledge, attitudes, and practice among Minnesota physicians. Minn Med. 2006;89(12 PG-50-54):50-54.
- Ross DS, Visser SN. Pediatric primary care physicians’ practices regarding newborn hearing screening. J Prim Care Community Health. 2012;3(4 PG-256-263):256-263.
- Elpers J, Lester C, Shinn JB, Bush ML. Rural family perspectives and experiences with early infant hearing detection and intervention: a qualitative study. J Community Health Publ Health Promot Dis Prev. 2016;41(2 PG-226-233):226-233. doi:10.1007/s10900-015-0086-1
- Gehring CE, Jones AL. Information given to parents of neonatal-intensive care unit graduates on hearing. J Early Hear Detect Interv. 2017;2(1 PG-29-39):29-39.
- Stich-Hennen JR, Bargen GA. Implementing a two class system for monitoring risk indicators for delayed-onset hearing loss. J Early Hear Detect Interv. 2017;2(1 PG-48-54):48-54.
- McInerney M, Scheperle R, Zeitlin W, Bodkin K, Uhl B. Adherence to follow-up recommendations for babies at risk for pediatric hearing loss. Int J Pediatr Otorhinolaryngol. 2020;132(PG-109900):109900.