Our Expanding Quality Program: Meeting the Needs of Our Diverse Specialty
In this our 125th anniversary year, it is appropriate that we celebrate our Quality Programs in this month’s Bulletin.
 James C. Denneny III, MD
James C. Denneny III, MD
AAO-HNS/F EVP/CEO
As we fast forward to the beginning of this century, the infrastructure was being developed by the Foundation under the leadership of David R. Nielsen, MD, in his role as Executive Vice President and CEO. The first decade produced a great deal of activity in the quality area and led to the development of our Guideline Task Force, which created a well-respected program for producing clinical practice guidelines (CPG) on critical patient care topics that have guided many physicians across many specialties in giving appropriate care to their patients. Guideline development led to performance measures that would become necessary for the PQRS quality reporting requirements instituted by CMS for Medicare.
The evolution of expectations related to quality healthcare advanced rapidly during the second decade, and it became apparent that quality would be an integral part of both governmental and private payer healthcare system models for the foreseeable future. To meet the challenge, the Foundation went through an extensive “due diligence” process for the purpose of establishing a clinical data registry (CDR). Subsequently, the Board of Directors authorized the use of reserve funding to create Reg-entSM, our clinical data registry. That registry, now entering its fifth year, has achieved both CDR and Qualified Clinical Data Registry (QCDR) status throughout its entire existence as designated by CMS.
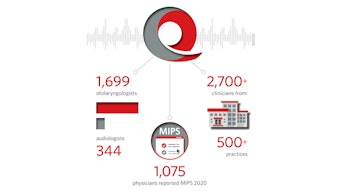
During the course of expanding the registry to measure quality for the breadth of our diverse specialty and maintaining our QCDR status, there have been a number of advancements. The AAO-HNSF has developed 22 specialty-specific quality measures over that time using several strategies to broaden our portfolio to ensure relevance across our specialty areas. We have pioneered innovative methodology to derive performance measures from existing CPGs and published the process in the Otolaryngology–Head and Neck Surgery. CMS has also attempted to improve the statutory MIPS quality program and hopes to debut their MIPS Value Pathways (MVP) in 2022 or 2023. When this program was announced, we created three novel examples that we have submitted for inclusion in the “pilot phase” and have recently met a second time to discuss this with CMS.
Like our Education and Meetings programs, our Quality and Research endeavors rely heavily on our dedicated member volunteers under the leadership of our Coordinator for Research and Quality and an extraordinary staff led by Senior Director, Jean Brereton. When you add the exceptional work of the Outcomes Research Evidence-Based Medicine (OREBM) and the Patient Safety and Quality Improvement (PSQI) Committees to the above-mentioned initiatives, our Quality programs remain on the cutting edge, serving our members’ current and future needs. The loop is closed by our practicing physicians as they participate in these various opportunities and incorporate information produced through these programs into their practices to the benefit of their patients across the spectrum of otolaryngic care.
Additionally, I would like to salute our forward-thinking members and practices who have chosen to participate and remain with Reg-ent through its growth and development. Without them we would not have been able to move forward to Phase II and our partnership with OM1 that will allow us to define “best care” in otolaryngology and participate in clinical research and trials that further define our goals.
I hope that you have noticed our new website, which was launched on June 7, 2021. Well over a year of planning and implementation were involved in this major project designed to freshen our branding and install a robust search engine that will result in a dramatic improvement over our predecessor platform. Our entire staff deserves a great deal of credit for bringing this vision to fruition in addition to their regular activities. Please give it a try and let us know what you think.










![05 Hpv Vaccination [converted]](https://img.ascendmedia.com/files/base/ascend/hh/image/2021/06/05_HPV_Vaccination__Converted_.60d0a1f25afe4.png?auto=format%2Ccompress&fit=crop&h=191&q=70&rect=45%2C0%2C1830%2C1830&w=340)