Milestone Moments
Each month, AAO-HNS highlights memorable moments in the history of the Academy.
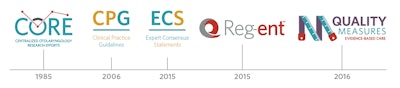
1985: The Centralized Otolaryngology Research Efforts (CORE) program was established.
2006: The development of quality knowledge products was led by the efforts of David R. Nielsen, MD, EVP/CEO, and Richard M. Rosenfeld, MD, MPH, MBA, Otolaryngology–Head and Neck Surgery Editor in Chief.
2006: The first set of measures on topics of Acute Otitis Externa and Otitis Media with Effusion was developed.
2006: Clinical Practice Guidelines: A Manual for Developing Evidence-Based Guidelines to Facilitate Performance Measurement and Quality Improvement was published.
2015: Clinical Consensus Statement (CCS) Development Manual was published. (The name change from CCSs to Expert Consensus Statements was approved in 2020.)
2015: The development of Reg-ent, a clinical data registry for otolaryngology-head and neck surgery, was approved.
2016: Reg-ent pilot was tested and launched, approved as a Qualified Clinical Data Registry and Qualified Registry by CMS and reported PQRS for 2016.
2016: Qualified Clinical Data Registry Measures Development began (To date, AAO-HNSF has developed 22 specialty-specific measures).
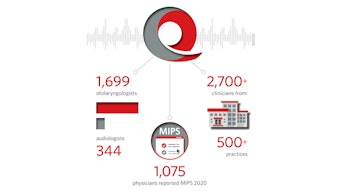
2017: Reg-ent launched Merit-Based Incentive Payment System (MIPS) reporting for its practices across all three categories: Quality, Improvement Activities, and Promoting Interoperability.
2020: Reg-ent began Phase II to serve as the basis for otolaryngology clinical research, to address product surveillance, and to provide a platform for internal and external research endeavors.
2021: Reg-ent launched the first Patient Reported Survey in Age-Related Hearing Loss.











![05 Hpv Vaccination [converted]](https://img.ascendmedia.com/files/base/ascend/hh/image/2021/06/05_HPV_Vaccination__Converted_.60d0a1f25afe4.png?auto=format%2Ccompress&fit=crop&h=191&q=70&rect=45%2C0%2C1830%2C1830&w=340)