Hidden Hearing Loss: State of the Science on Testing
The Hearing Committee addresses HHL and treatment strategies to prevent further permanent damages.
Mehdi Abouzari, MD, PhD, Harrison W. Lin, MD, and Hamid R. Djalilian, MD, Hearing Committee members
A small percentage of adult patients complaining of hearing loss have normal pure tone audiograms.1 These patients often have difficulty hearing in noisy environments. This phenomenon has been termed “hidden hearing loss” (HHL), where the patient complains of a hearing deficit but the audiogram is normal and thus the hearing loss is “hidden.” The understanding of the potential cause of HHL was first described in animals by Liberman and Kujawa.2,3 The two scientists described the underlying histopathologic correlate known as cochlear synaptopathy. In cochlear synaptopathy, there is damage to and loss of synapses between the hair cells and the auditory nerve fibers.
HHL in humans is characterized by difficulties in speech discrimination and intelligibility in noisy environments in a patient with a normal audiogram. HHL can occur as a result of noise exposure, aging, or peripheral neuropathies.4 Exposures that lead to only a temporary rise in auditory thresholds can cause damage to the fibers in the auditory nerve conveying sound information to the brain. Such damage may not affect the detection of tones, as shown on the audiogram, but it can hamper the ability to process more complex signals.
At least four neurophysiological pathologies impair the encoding of sounds without elevating hearing thresholds, including cochlear synaptopathy, auditory nerve demyelination, elevated neural gain in the central nervous system, and impaired neural adaptation.5 Since hair cells are intact in this condition, patients often preserve normal hearing thresholds; however, due to synaptic loss, it has been thought that they may experience hearing difficulties in the presence of noise and experience tinnitus and hyperacusis.2
The most frequently used test for HHL diagnosis is the auditory brainstem response (ABR) analysis. In the animal model, HHL can be identified by a characteristic reduction in wave I peak amplitude in the absence of ABR threshold elevation or latency changes. Wave I also represents amplitudes that can be correlated with the degree of cochlear synaptopathy in animal models.6 Unlike in animal models, ABR waves in humans vary by sex, age, stimulus, and recording methods, limiting their clinical application for routine HHL diagnosis in individual patients.7 Moreover, it has been shown that low signal-to-noise ratios in noninvasive measurement of the ABR limit its clinical utility in diagnosing cochlear synaptopathy in humans.8 Therefore, in human listeners, the wave I/wave V amplitude ratio has been proposed as an indicator of elevated central gain—a relative measure within the individual that is intended to reduce inter-subject variability and is based on the hypothesis that cochlear synaptopathy generates a reduced amplitude wave I and a compensatory increase in wave V amplitude in audiometrically normal individuals with tinnitus (for whom the term HHL was originally coined).9 Recently, a study has suggested that masking noise on the ABR wave V latency might be a better indicator of underlying synaptic damage.10
Table 1. Auditory Brainstem Response (ABR) Electric Signature and hearing status.

The second diagnostic tool proposed from animal models is the envelope-following response (EFR). The EFR is an auditory steady-state response evoked by amplitude modulation tones and has been used as a physiological measure of the temporal representation of suprathreshold sounds in the auditory brain.11 Louder sounds than threshold are used for the EFR because it has been found that noise preferentially causes damage to a subset of auditory nerve fibers with higher thresholds.6 In contrast to transient tones used in ABR studies to evoke electric responses, the amplitude modulated tones in the EFR are continuous and sinusoidal (Figure 1). It has been shown that EFR deficits are correlated with reduced signal detection in noise and a more reliable signature for cochlear synaptopathy since EFR changes are larger than ABR amplitude reductions in the same animal model.12-14
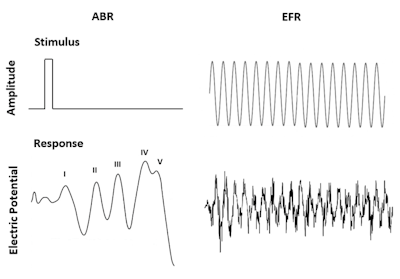 Figure 1. Comparison of typical stimuli and recorded waveforms (response) for ABR and EFR.
Figure 1. Comparison of typical stimuli and recorded waveforms (response) for ABR and EFR.
Recent animal studies showed that EFR deficits to amplitude modulation frequencies near 1 kHz highly correlated with synaptic loss and noise- or age-induced HHL.13,14 Similarly, in a human study, control subjects had better EFRs to a 5 kHz amplitude modulated tone at 85 Hz in the background noise compared with subjects with suspected HHL whose EFRs were more affected by noise.15 These comparative human and animal studies support the use of EFR as a potential metric for cochlear synaptopathy and HHL. Clinical software for EFR is currently not available, and these studies are performed in research settings.
Finally, the stapedial reflex, also known as middle ear muscle reflex (MEMR), has been proposed as a potential method of testing for HHL due to its strong dependence on the integrity of the high-threshold auditory nerve afferent fibers. MEMR is measured by observing sound pressure changes in the ear canal in response to an emitted tone in the ipsilateral or contralateral ear. Loud sounds contract the stapedius muscle, stiffening the ossicular chain and tilting the stapes away from the cochlea. This elicits a bilateral increase in middle ear impedance due to stapedial muscle contraction.
The MEMR responses are significantly lower in patients with tinnitus and normal hearing thresholds than control subjects without tinnitus, which may reflect the presence of HHL.16 The MEMR thresholds of humans were correlated with word recognition scores in difficult listening environments, suggesting that they may be a better predictor of primary neural degeneration compared with wave I ABR amplitude reduction.17
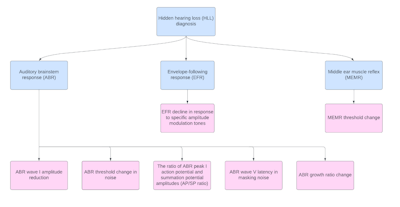 Figure 2. An algorithm for different diagnostic tools and their respective electrical measures representative of hidden hearing loss.
Figure 2. An algorithm for different diagnostic tools and their respective electrical measures representative of hidden hearing loss.
Click here for larger image.
Computational modeling and auditory nerve imaging are the other two approaches for diagnosis of cochlear synaptopathy. The computational models usually build on several physiological assumption, which make them less reliable. Moreover, these models cannot determine the actual synaptic loss for an individual. Auditory nerve imaging (e.g., high-field MRI or diffusion tension imaging MRI) is not a cost-effective option for routine use in the clinic as a diagnostic test. Also, it may take months or years after the time of initial lesion for the cochlear nerve to atrophy to such an extent that the effects of synaptopathy are measurable using imaging techniques.18 There are little available data relating synaptopathy to behavioral performance. Noise exposure and aging have been related to deficits in temporal processing tasks and speech discrimination in noise.19 For HHL, ultra-high-frequency audiometry may help identify people with sensory hair cell loss that does not appear on standard audiograms.20 However, these behavioral measures require a response from the listener thus not an objective diagnostic tool for HHL detection. Figure 2 provides an algorithm for diagnostic tools.
Further work should be directed at developing precise, sensitive, and reproducible noninvasive diagnostic techniques to detect cochlear synaptopathy (i.e., hidden hearing loss). Such tools will help clinicians and researchers to understand the frequency and natural history of HHL, to identify the responsible etiologies in each patient, and eventually to validate the treatment strategies for this condition to prevent further permanent damages.
References
- Hind SE, Haines-Bazrafshan R, Benton CL, Brassington W, Towle B, Moore DR. Prevalence of clinical referrals having hearing thresholds within normal limits. Int J Audiol. 2011;50:708-716.
- Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res. 2017;349:138-147.
- Lin HW, Furman AC, Kujawa SG, Liberman MC. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605-616.
- Kohrman DC, Wan G, Cassinotti L, Corfas G. Hidden hearing loss: a disorder with multiple etiologies and mechanisms. Cold Spring Harb Perspect Med. 2020;10:a035493.
- Valderrama JT, de la Torre A, McAlpine D. The hunt for hidden hearing loss in humans: from preclinical studies to effective interventions. Front Neurosci. 2022;16:1000304.
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577-586.
- Bajin MD, Dahm V, Lin VYW. Hidden hearing loss: current concepts. Curr Opin Otolaryngol Head Neck Surg. 2022;30:321-325.
- Turner K, Moshtaghi O, Saez N, Richardson M, Djalilian H, Zeng FG, Lin H. Auditory brainstem response wave I amplitude has limited clinical utility in diagnosing tinnitus in humans. Brain Sci. 2022;12:142.
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452-13457.
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci. 2016;36:3755-3764.
- Bharadwaj HM, Verhulst S, Shaheen L, Liberman MC, Shinn-Cunningham BG. Cochlear neuropathy and the coding of supra-threshold sound. Front Syst Neurosci. 2014;8:26.
- Dimitrijevic A, John MS, Picton TW. Auditory steady-state responses and word recognition scores in normal-hearing and hearing-impaired adults. Ear Hear. 2004;25:68-84.
- Parthasarathy A, Kujawa SG. Synaptopathy in the aging cochlea: characterizing early-neural deficits in auditory temporal envelope processing. J Neurosci. 2018;38:71087119.
- Shaheen LA, Valero MD, Liberman MC. Towards a diagnosis of cochlear neuropathy with envelope following responses. J Assoc Res Otolaryngol. 2015;16:727-745.
- Paul BT, Bruce IC, Roberts LE. Evidence that hidden hearing loss underlies amplitude modulation encoding deficits in individuals with and without tinnitus. Hear Res. 2017;344:170-182.
- Wojtczak M, Beim JA, Oxenham AJ. Weak middle-ear-muscle reflex in humans with noise-induced tinnitus and normal hearing may reflect cochlear synaptopathy. eNeuro. 2017;4:ENEURO.0363-17.2017.
- Mepani AM, Kirk SA, Hancock KE, Bennett K, de Gruttola V, Liberman MC, Maison SF. Middle ear muscle reflex and word recognition in ”normal-hearing” adults: evidence for cochlear synaptopathy? Ear Hear. 2020;41:25–38.
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG. Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686-13694.
- Plack CJ, Léger A, Prendergast G, Kluk K, Guest H, Munro KJ. Toward a diagnostic test for hidden hearing loss. Trends Hear. 2016;20:2331216516657466.
- Barbee CM, James JA, Park JH, Smith EM, Johnson CE, Clifton S, Danhauer JL. Effectiveness of auditory measures for detecting hidden hearing loss and/or cochlear synaptopathy: a systematic review. Semin Hear. 2018;39:172-209.