2013 Annual Report: Research and Quality
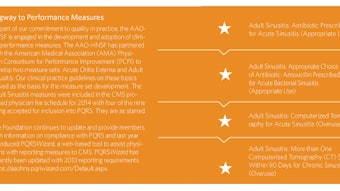
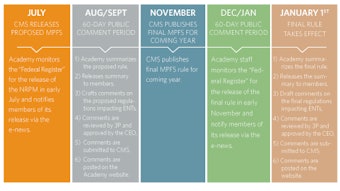
To further research and quality improvement in the field of otolaryngology, our goal is to empower physicians to provide the best patient care through the development of evidence-based clinical practice guidelines. We look to identify, promote, and address the key research questions and disseminate discoveries for advancement in our field and to fundamentally improve patient outcomes. This year, with coordinator John S. Rhee, MD, MPH, we have progressed to meet these strategies: Build a sustainable infrastructure to test, pilot, and promote adoption of research and quality products such as guidelines, measures, and evidence-based medicine to promote translational research. Build and promote a strong research-granting program for the specialty. In addition, the first clinical practice guideline developed by the AAO-HNSF, Acute Otitis Externa, has been updated and will be published in February 2014. The AAO-HNSF has spent the past two years working to update its processes to ensure compliance to the 2011 Institute of Medicine report entitled Clinical Practice Guidelines We Can Trust and the 2012 publication of the Guidelines International Network: International Standards for Clinical Practice Guidelines. As a result, the third edition of the Clinical Practice Guideline Development Manual: A Quality Driven Approach for Translating Evidence into Action was updated to document the AAO-HNSF compliance to these new standards and published in January 2013. The AAO-HNSF published five new quality knowledge products: Clinical Consensus Statement: Appropriate Use of CT for Paranasal Sinus Disease (November 2012) Clinical Consensus Statement: Tracheostomy Care (January 2013) Clinical Practice Guideline: Improving Voice Outcomes after Thyroid Surgery (June 2013) Clinical Practice Guideline: Tympanostomy Tubes in Children (July 2013) Clinical Practice Guideline: Bell’s Palsy (November 2013) Clinical Practice Guideline: Acute Otitis Externa (February 2014)
 Drs. Reginal Baugh, Lisa Ishii, and Gregory Basura presented during the 2013 Annual Meeting.
Drs. Reginal Baugh, Lisa Ishii, and Gregory Basura presented during the 2013 Annual Meeting.To further research and quality improvement in the field of otolaryngology, our goal is to empower physicians to provide the best patient care through the development of evidence-based clinical practice guidelines. We look to identify, promote, and address the key research questions and disseminate discoveries for advancement in our field and to fundamentally improve patient outcomes. This year, with coordinator John S. Rhee, MD, MPH, we have progressed to meet these strategies:
- Build a sustainable infrastructure to test, pilot, and promote adoption of research and quality products such as guidelines, measures, and evidence-based medicine to promote translational research.
- Build and promote a strong research-granting program for the specialty.
In addition, the first clinical practice guideline developed by the AAO-HNSF, Acute Otitis Externa, has been updated and will be published in February 2014. The AAO-HNSF has spent the past two years working to update its processes to ensure compliance to the 2011 Institute of Medicine report entitled Clinical Practice Guidelines We Can Trust and the 2012 publication of the Guidelines International Network: International Standards for Clinical Practice Guidelines. As a result, the third edition of the Clinical Practice Guideline Development Manual: A Quality Driven Approach for Translating Evidence into Action was updated to document the AAO-HNSF compliance to these new standards and published in January 2013.
- Clinical Consensus Statement: Appropriate Use of CT for Paranasal Sinus Disease (November 2012)
- Clinical Consensus Statement: Tracheostomy Care (January 2013)
- Clinical Practice Guideline: Improving Voice Outcomes after Thyroid Surgery (June 2013)
- Clinical Practice Guideline: Tympanostomy Tubes in Children (July 2013)
- Clinical Practice Guideline: Bell’s Palsy (November 2013)
- Clinical Practice Guideline: Acute Otitis Externa (February 2014)