Craniomaxillofacial Surgery
Otolaryngology play an essential role in the field of craniomaxillofacial surgery.
Florence Othieno, MD, and Sherard Tatum, MD, President, American Academy of Facial Plastic and Reconstructive Surgery
Craniomaxillofacial or craniofacial surgery is a relatively modern surgical discipline. These terms are often used interchangeably. Use of “craniofacial” to refer to cranio-orbital problems and “craniomaxillofacial” to include the mid and lower facial skeleton can be helpful, but this is far from generally accepted.
Although modern craniomaxillofacial surgery started in the mid-1800s, evidence of different forms of cranial surgery and craniofacial deformation dates to 12,000 BCE. Archeologists have found evidence of skull surgery performed in the Neolithic Age. Archeologic findings have revealed healed surgical trephinations in human skulls with craniosynostosis. Trephinations were also performed in many parts of the world to treat nonsurgical conditions, for example, epilepsy and intractable headaches.1 Intentional cranial deformation was also widespread in the West Indies, Cuba, South America, Europe, and ancient Egypt. For example, ancient Egyptian Queen Nefertiti’s head was bound after placing a stone at the nape of her neck to produce her elongated head shape.2
Multiple subspecialties, including otolaryngology, neurosurgery, plastic surgery, oculoplastic surgery, and maxillofacial surgery, have played essential roles in the evolution of craniomaxillofacial surgery. The first orthognathic surgery was performed by an American surgeon, Simon Hullihen, in 1849.3 He reported a case of surgical correction of a prognathic mandibular open bite deformity secondary to severe scar contractures from neck and chest burns. He performed a staged reconstruction, including a V-shaped wedge ostectomy and setback of the anterior mandible via an intraoral approach. The scar and lip were revised in subsequent procedures. In the 1940s and 1950s, German maxillofacial surgeons were doing percutaneous supraforaminal (above the mandibular foramen) ramus osteotomies (Blair or Kostecka procedure) with local anesthesia and a Gigli saw in a dental chair. Subsequently, Hugo Obwegeser developed the sagittal split ramus osteotomy in 1953.4
The first maxillary procedure reported was a unilateral Le Fort I type (not called as such at the time) osteotomy performed in 1859 by Bernhard von Langenbeck in Germany and in 1867 by David Cheever at Boston City Hospital. The approach provided access for the removal of a nasopharyngeal tumor in an 18-year-old student with a two-year history of epistaxis. A few years later, Cheever performed a bilateral Le Fort I osteotomy on a 41-year-old with a posterior nasal cavity mass.5 In 1901 the French surgeon René Le Fort described the natural planes of midface fractures after experiments using blunt trauma from different directions to intact cadaveric faces. The goal of the study at the time was to prove that traumatic midface fractures did not radiate to the skull base. The terminology Le Fort I, II, and III osteotomies derived from their similarity to the Le Fort fractures.6 In 1927 Martin Wassmund performed a maxillary osteotomy at the Le Fort I level without pterygoid plate disjunction to correct a post-traumatic open bite with elastics. He used the traction to elongate the anterior maxilla postoperatively; unfortunately, the maxilla eventually relapsed. After attempts by other surgeons, the modern Le Fort I osteotomy was described by Obwegeser, who believed pterygomaxillary disjunction with full mobilizing of the maxilla was critical for the success of the procedure. He also suggested using autologous bone grafts between the pterygoid plates and maxillary tuberosities to aid with healing.3,4
The first recorded approaches for surgical correction of craniosynostosis were performed in 1890 and 1892 by two neurosurgeons, Odilon Marc Lannelogue and L. C. Lane, respectively. Lannelogue advocated for the release but not resection of the fused cranial suture. Lane performed the first strip craniotomy two years later.7 In the late 1960s and early 1970s aesthetic objectives began to receive emphasis. Over the past several decades, craniofacial surgery has developed its own identity, with Paul Tessier being credited as the founding father. Sir Harold Gilles, an otolaryngologist trained by the French oral surgeon Charles Valadier, performed the first Le Fort III surgery in 1951 on a patient with midface retrusion; unfortunately, the patient eventually relapsed. The technique was later revived by Tessier, who used bone grafts to achieve stability. Tessier presented the results of his cases in 1967, demonstrating improved quality of life for those with severe disfigurements due to underlying craniofacial dysostoses, for example, Crouzon, Treacher Collins, and Apert. He also made five different variants of the lateral orbital osteotomies.7 This opened a world of possibilities for the management of other malformation syndromes affecting the skull and face, leading to the inception of the new specialty of craniofacial surgery.
In 1975 Fernando Ortiz Monasterio noted that many craniofacial deformities were associated with maxillary hypoplasia. He combined bilateral fronto-orbital advancement with Le Fort III advancement in the monobloc osteotomy, allowing the advancement of the orbits and midface in a single segment. He used bone grafts to maintain the advancement. A year later J. C. van der Meulen described the possibility of simultaneous correction of hypertelorism and fronto-facial deficiencies. He performed the facial bipartition surgery in 1979, simultaneously correcting the patient’s orbital hypertelorism and midface deformity by splitting the monobloc, resecting central bone, narrowing the interorbital distance, and correcting the plane of the hard palate.8,9 In more recent years, distraction has been used to advance the monobloc reducing the connection of the sinonasal cavities with cranial cavity. Distraction osteogenesis was first described by Joseph McCarthy in 1992. He performed gradual distraction in patients with Nager syndrome, lengthening the mandible from 21 mm in approximately nine weeks.10 A year later Constantino et al. described a series of mandibular elongations using distraction osteogenesis.11
Principles
The main objectives of craniomaxillofacial surgery are to improve function and aesthetics. Fundamental principles of craniomaxillofacial surgery include precision, proper and timely surgery, and vision. A clear vision of current and long-term goals is imperative because growth changes must be considered. As facial aesthetics are improved, function should not be ignored. Appreciation for bone physiology and anatomy, as well as the awareness of bone stock availability, is critical when planning craniomaxillofacial procedures. The underlying bone's biomechanical properties should withstand the soft tissue's elastic recoil. The interrelationships between multiple anatomic areas, proper alignment, and growth should also be carefully considered. It is not unusual for patients with craniomaxillofacial anomalies to undergo multiple staged surgeries during childhood and adolescence. In many cases, definitive repair of skeletal abnormalities is deferred until completion of facial growth to allow for predictable and stable results. Early intervention is, at times, warranted to prevent adverse sequelae such as brain and ocular injuries or airway problems. Surgery typically begins with wide skeletal exposure prior to any osteotomies. Once the bone segments are mobilized, appropriate rigid internal fixation is utilized. Bone grafting is used to maintain stability of large movements. For even larger movements, distraction can be implemented to prevent relapse. Endoscopic and minimally invasive approaches have become popular over the past several years.
Applications
Craniomaxillofacial surgeons apply these principles of facial skeletal surgery to repair congenital head and neck anomalies, trauma, and post-ablative defects. Syndromic congenital defects tend to be more complex, requiring multiple interventions prior to adulthood. In craniosynostosis, cranial vault remodeling (Figure 1) is performed to improve aesthetics and enlarge the intracranial and orbital volumes as needed to prevent adverse sequelae. Timing for intervention is controversial, with significant variability across craniomaxillofacial centers. While early intervention can prevent sequelae of increased intracranial pressure and corneal exposure, proponents for later surgery cite a more stable bone correction with less need for subsequent revision. The surgical approach, determined by a surgeon’s expertise, can either be endoscopic or open. Endoscopic approaches involve limited access incisions with suturectomy of the affected suture along with wedge osteotomies or barrel staves in areas of constriction. Distractors or springs can be used to separate bone segments. Postoperative helmet therapy is frequently used after endoscopic approaches to limit growth in the undesirable growth vectors. Coronal or sagittal incisions are used in most open approaches.12,13
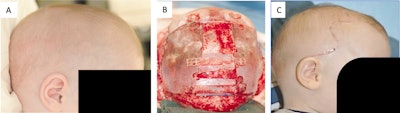
Figure 1. Cranial vault remodeling. (A) Preoperative view of sagittal synostosis showing scaphocephaly with increased anteroposterior (AP) diameter, biparietal narrowing, and frontal bossing. (B) Subtotal cranial‐vault remodeling intraoperative view, patient supine, wavy coronal incision with scalp flaps reflected anteriorly and posteriorly. Frontal bone transposition has been performed, the parietal bones have been widened, and the central gap has been grafted with prelambdoid bone. Note the persistent prelambdoid defect that will allow the occipital cone to come forward with supine sleep position. (C) Postoperative view after PCVR showing wavy coronal incision, improved AP diameter, and improved frontal aesthetics. From: Tatum SA, et al. Differential management of scaphocephaly. Laryngoscope. 2012;122[2]:246-253.13
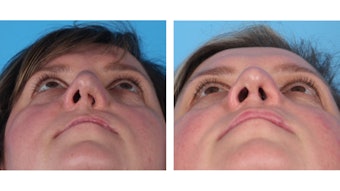
Treacher Collins syndrome (TCS), also known as mandibulofacial dysostosis, is an autosomal dominant disorder with characteristic dysmorphic orbits, down slanting palpebral fissures; hypoplasia of the mandible, zygoma, and palate; and middle-ear deformities. The degree of the defects can range from mild to severe. Given the complexity of TCS defects, multiple staged repairs are often warranted, with airway issues addressed first, frequently with tracheostomy or distraction. A gastrostomy tube may be placed in cases where feeding is an issue due to severe micrognathia. The micrognathic mandible can safely be lengthened with distraction osteogenesis in early childhood if needed (Figure 2).14 In cases with stable airways and limited obstructive symptoms, waiting as long as possible reduces the risk of dental damage, TMJ dysfunction, and multiple distractions prior to skeletal maturation. Midface hypoplasia is typically repaired using a Le Fort procedure after skeletal maturity is achieved. Distraction in TCS is commonly associated with high relapse rates due to inadequate bone stock secondary to bony hypoplasia and chronic myofascial contraction.15
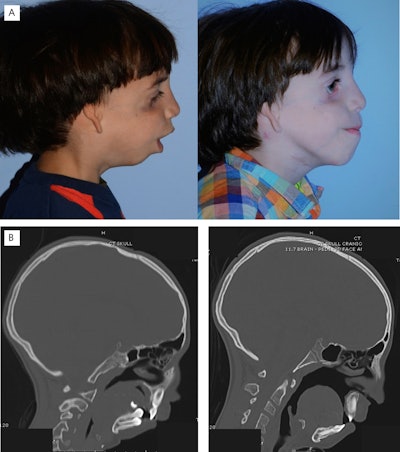
Figure 2. Micrognathic mandible lengthening with distraction osteogenesis. (A) Preoperative (left) and postoperative (right) clinical photographs of a patient with Treacher Collins following mandibular distraction osteogenesis. (B) Pre-distraction (left) and post-distraction (right) CT showing significant decrease in airway obstruction. From: Peck CJ, et al. Treacher Collins mandibular distraction. Clin Plast Surg. Jul 2021;48[3]:431-444.
Otolaryngology Belongs in CMF Surgery
Multiple subspecialties have contributed to the evolution of modern-day craniomaxillofacial surgery. The diversity of surgical backgrounds has propelled the field forward. As otolaryngologists, we have acquired regional-based specialization and are experts in head and neck anatomy and physiology. Our expertise, familiarity with ancillary services—including speech pathology and audiology—and frequent interaction with other specialties such as ophthalmology, neurosurgery, and oral and maxillofacial surgery, allow us to play an essential role in the field of craniomaxillofacial surgery.
References
- Goodrich JT, Tutino M. An annotated history of craniofacial surgery and intentional cranial deformation. Neurosurg Clin N Am. Jan 2001;12(1):45-68, viii.
- Snorrason E. Cranial deformation in the reign of Akhnaton. Bull Hist Med. Dec 1946;20(5):601-610.
- Aziz SR. Simon P. Hullihen and the origin of orthognathic surgery. J Oral Maxillofac Surg. Oct 2004;62(10):1303-1307. doi:10.1016/j.joms.2003.08.044
- Obwegeser HL. Orthognathic surgery and a tale of how three procedures came to be: a letter to the next generations of surgeons. Clin Plast Surg. Jul 2007;34(3):331-355. doi: 10.1016/j.cps.2007.05.014. PMID: 17692696.
- Halvorson EG, Mulliken JB. Cheever's double operation: the first Le Fort I osteotomy. Plast Reconstr Surg. Apr 2008;121(4):1375-1381. doi:10.1097/01.prs.0000304442.15532.40
- Noffze MJ, Tubbs RS. Rene Le Fort 1869-1951. Clin Anat. Apr 2011;24(3):278-81. doi:10.1002/ca.21091
- Clayman MA, Murad GJ, Steele MH, Seagle MB, Pincus DW. History of craniosynostosis surgery and the evolution of minimally invasive endoscopic techniques: the University of Florida experience. Ann Plast Surg. Mar 2007;58(3):285-287. doi:10.1097/01.sap.0000250846.12958.05
- van der Meulen JC. Medial faciotomy. Br J Plast Surg. Oct 1979;32(4):339-342. doi:10.1016/0007-1226(79)90095-x
- Batut C, Joly A, Travers N, Guichard B, Pare A, Laure B. Surgical treatment of orbital hypertelorism: historical evolution and development prospects. J Craniomaxillofac Surg. Nov 2019;47(11):1712-1719. doi:10.1016/j.jcms.2019.07.002
- McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plas Reconstr Surg. Jan 1992;89(1):1-8; discussion 9-10.
- Costantino PD, Friedman CD. Distraction osteogenesis. Applications for mandibular regrowth. Otolaryngol Clin North Am. Dec 1991;24(6):1433-1443. PMID: 1792079
- Xue AS, Buchanan EP, Hollier LH Jr. Update in management of craniosynostosis. Plast Reconstr Surg. Jun 1 2022;149(6):1209e-1223e. doi:10.1097/PRS.0000000000009046
- Tatum SA, Jones LR, Cho M, Sandhu RS. Differential management of scaphocephaly. Laryngoscope. Feb 2012;122(2):246-53. doi:10.1002/lary.22463
- Plomp RG, van Lieshout MJS, Joosten KFM, et al. Treacher Collins syndrome: a systematic review of evidence-based treatment and recommendations. Plast Reconstr Surg. Jan 2016;137(1):191-204. doi:10.1097/PRS.0000000000001896
- Peck CJ, Lopez J, Smetona JT, Steinbacher DM. Treacher Collins mandibular distraction. Clin Plast Surg. Jul 2021;48(3):431-444. doi:10.1016/j.cps.2021.02.005