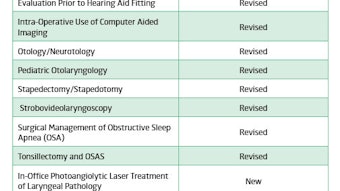
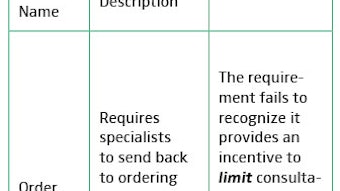
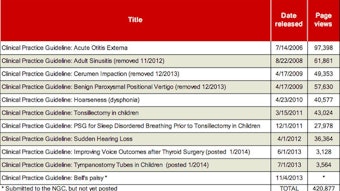
History of the CHEER Clinical Research Coordinator Conference – EXTENDED ONLINE VERSION
Shaun A. Nguyen, MD, MA, CPI The history of clinical research emphasizes the close relationship between clinical research and clinical care. The ethics of clinical research tend to focus on whether, and to what extent, the treatment of research subjects diverges from the norms of clinical care. Clinical research is often conducted with patients and is often performed by physicians and clinical research coordinators. Modern clinical research studies are accompanied by more rules, regulations, laws, and guidelines than ever governing research with human subjects. I am director of Clinical Research for the department of otolaryngology-head and neck surgery at the Medical University of South Carolina (MUSC), where we are training a new generation of otolaryngology physician investigators through our research fellowship program. In addition, through the collaboration with the Master of Science in Clinical Research Program at the Medical University of South Carolina, we are providing an internship program for clinical research coordinators with more than 30 investigator-initiated and corporate-sponsored clinical trials. MUSC research fellows and clinical research coordinators have attended the Annual CHEER Conference since its inception in 2008. The two-day Annual CHEER Coordinator’s Conference provides physician investigators and clinical research coordinators the knowledge and expertise to conduct clinical research studies and follow principles of good clinical practice (GCP). The conference delivers disease-specific education and an overview of the clinical research process: its function, history, and development. Physician-investigators, research fellows, and clinical research coordinators learn project management skills and clinical practice guidelines. They learn about research team member roles, typical study designs, and developmental phases of clinical trials. For the 6th Annual CHEER Conference, MUSC sent three research fellows and three students for training. Conference Overview P. Ryan Camilon The 6th annual CHEER conference differed from previous meetings by greater incorporation of disease-specific education among the usual clinical-trial focused lectures. Neurotologist Alan Langman, MD, provided an overview of the pathophysiology, evaluation, and treatment of Meniere’s disease in the context of the aspects currently being explored. Melissa Pynnonen, MD, explained several issues in the diagnosis and treatment of sinusitis as well as challenges that need to be tackled concerning sinusitis research. Laryngologist Joshua Schindler, MD, presented a summary of laryngeal pathology after voice therapist Donna Graville, PhD, discussed possible areas of investigation in voice management. Finally, neurotologist Debara Tucci, MD, MS, (Co-PI for CHEER) reviewed sudden sensorineural hearing loss and current treatment controversies. Typically, the CHEER conference focuses heavily on research-practical topics, which were still thoroughly addressed. David Witsell, MD, MHS, (CHEER PI) and Kris Schulz, MPH (Director of Research), summarized CHEER’s current clinical trials and discussed the growth of the network, which now consists of 28 sites in 18 states. Select CHEER research coordinators presented topics covering FDA investigator qualification, new methods of site monitoring, preparation for an FDA audit, and IRB regulatory documentation. Also new in this year’s conference was an emphasis on the open discussion of ideas and experiences between coordinators. This new theme proved to be rewarding. Small-group sessions on how to ethically get consent from patients and properly manage their information led to the sharing of troubleshooting tips and methods to improve researcher technique. This year, CHEER provided coordinators with a better understanding of ear, nose, and throat pathology in addition to covering the latest changes in the research regulatory landscape. By presenting coordinators with more medical information, we hope to ensure a greater understanding of how CHEER projects will improve the treatment of patients, and also foresight into what aspects of head and neck pathology may be explored down the road. Reflection on the Experience Colin Fuller, MD, MS While we were all excited to be invited to participate in the annual CHEER Conference, I admit that I was unsure what to expect from the meeting. As a research fellow coordinating clinical trials for the first time, I anticipated a top-down, hierarchical situation where PIs would dole out instructions on how to facilitate practice-based research in general, and how to conduct the joint CHEER trials specifically. Instead, we were invited to an open discussion with providers at every level (PIs, nurses, SLPs, audiologists, residents, and coordinators) speaking directly about the conduct of clinical research. From Dr. Witsell’s opening address on the first day, a collegial and friendly atmosphere permeated every lecture and discussion. We were encouraged to contribute to the development of specific research protocol for the CHEER network studies, which helped to make the data collection process as efficient and practical as possible. We also shared ideas for how to improve data collection and subject protection for all human research studies. These discussions were incredibly informative to us as newly minted research fellows. Dr. Witsell’s remarks urged us to strive to replicate the behaviors of the most successful among us—to be, or to emulate, the “positive deviant.” With the conference bringing to bear the experiences and resources of so many research professionals, the CHEER network will be well-served by this philosophy. In the near future, I am quite confident that other specialties will be emulating the CHEER network itself as that “positive deviant.” How Does this Prepare Me for the Future (As a Future PI)? Amit J. Sood In addition to direct clinical care, my future practice as an academic otolaryngologist will include contributing to advancements in patient care. Clinical research, as a vehicle for medical advancement, enables a provider to treat patients with cutting-edge technologies and the most up-to-date knowledge of disease processes. The CHEER conference offers many learning opportunities for those interested in clinical research. The CHEER network fosters an appreciation for clinically driven research in an informal setting for providers at every level, including aspiring PIs like myself. In addition to informative clinical presentations, the CHEER network discusses the inner workings of “what to expect” when undertaking a clinical trial from start to finish. Experienced coordinators explain concepts such as IRB process, protocol design, and funding, while others provide insight on the “pitfalls” of clinical trials such as FDA audits. The conference instills feelings of appreciation for clinically oriented research and a greater desire to perform clinical research to enhance patient care for the next generation of patients. Lastly, it provides specific tools, connections, and resources that are needed to help facilitate successful clinical trials for coordinators and future PIs alike.
Shaun A. Nguyen, MD, MA, CPI
The history of clinical research emphasizes the close relationship between clinical research and clinical care. The ethics of clinical research tend to focus on whether, and to what extent, the treatment of research subjects diverges from the norms of clinical care. Clinical research is often conducted with patients and is often performed by physicians and clinical research coordinators. Modern clinical research studies are accompanied by more rules, regulations, laws, and guidelines than ever governing research with human subjects.
I am director of Clinical Research for the department of otolaryngology-head and neck surgery at the Medical University of South Carolina (MUSC), where we are training a new generation of otolaryngology physician investigators through our research fellowship program. In addition, through the collaboration with the Master of Science in Clinical Research Program at the Medical University of South Carolina, we are providing an internship program for clinical research coordinators with more than 30 investigator-initiated and corporate-sponsored clinical trials. MUSC research fellows and clinical research coordinators have attended the Annual CHEER Conference since its inception in 2008. The two-day Annual CHEER Coordinator’s Conference provides physician investigators and clinical research coordinators the knowledge and expertise to conduct clinical research studies and follow principles of good clinical practice (GCP). The conference delivers disease-specific education and an overview of the clinical research process: its function, history, and development. Physician-investigators, research fellows, and clinical research coordinators learn project management skills and clinical practice guidelines. They learn about research team member roles, typical study designs, and developmental phases of clinical trials. For the 6th Annual CHEER Conference, MUSC sent three research fellows and three students for training.
Conference Overview
P. Ryan Camilon
The 6th annual CHEER conference differed from previous meetings by greater incorporation of disease-specific education among the usual clinical-trial focused lectures. Neurotologist Alan Langman, MD, provided an overview of the pathophysiology, evaluation, and treatment of Meniere’s disease in the context of the aspects currently being explored. Melissa Pynnonen, MD, explained several issues in the diagnosis and treatment of sinusitis as well as challenges that need to be tackled concerning sinusitis research. Laryngologist Joshua Schindler, MD, presented a summary of laryngeal pathology after voice therapist Donna Graville, PhD, discussed possible areas of investigation in voice management. Finally, neurotologist Debara Tucci, MD, MS, (Co-PI for CHEER) reviewed sudden sensorineural hearing loss and current treatment controversies.
Typically, the CHEER conference focuses heavily on research-practical topics, which were still thoroughly addressed. David Witsell, MD, MHS, (CHEER PI) and Kris Schulz, MPH (Director of Research), summarized CHEER’s current clinical trials and discussed the growth of the network, which now consists of 28 sites in 18 states. Select CHEER research coordinators presented topics covering FDA investigator qualification, new methods of site monitoring, preparation for an FDA audit, and IRB regulatory documentation. Also new in this year’s conference was an emphasis on the open discussion of ideas and experiences between coordinators. This new theme proved to be rewarding. Small-group sessions on how to ethically get consent from patients and properly manage their information led to the sharing of troubleshooting tips and methods to improve researcher technique.
This year, CHEER provided coordinators with a better understanding of ear, nose, and throat pathology in addition to covering the latest changes in the research regulatory landscape. By presenting coordinators with more medical information, we hope to ensure a greater understanding of how CHEER projects will improve the treatment of patients, and also foresight into what aspects of head and neck pathology may be explored down the road.
Reflection on the Experience
Colin Fuller, MD, MS
While we were all excited to be invited to participate in the annual CHEER Conference, I admit that I was unsure what to expect from the meeting. As a research fellow coordinating clinical trials for the first time, I anticipated a top-down, hierarchical situation where PIs would dole out instructions on how to facilitate practice-based research in general, and how to conduct the joint CHEER trials specifically.
Instead, we were invited to an open discussion with providers at every level (PIs, nurses, SLPs, audiologists, residents, and coordinators) speaking directly about the conduct of clinical research. From Dr. Witsell’s opening address on the first day, a collegial and friendly atmosphere permeated every lecture and discussion. We were encouraged to contribute to the development of specific research protocol for the CHEER network studies, which helped to make the data collection process as efficient and practical as possible. We also shared ideas for how to improve data collection and subject protection for all human research studies. These discussions were incredibly informative to us as newly minted research fellows.
Dr. Witsell’s remarks urged us to strive to replicate the behaviors of the most successful among us—to be, or to emulate, the “positive deviant.” With the conference bringing to bear the experiences and resources of so many research professionals, the CHEER network will be well-served by this philosophy. In the near future, I am quite confident that other specialties will be emulating the CHEER network itself as that “positive deviant.”
How Does this Prepare Me for the Future (As a Future PI)?
Amit J. Sood
In addition to direct clinical care, my future practice as an academic otolaryngologist will include contributing to advancements in patient care. Clinical research, as a vehicle for medical advancement, enables a provider to treat patients with cutting-edge technologies and the most up-to-date knowledge of disease processes.
The CHEER conference offers many learning opportunities for those interested in clinical research. The CHEER network fosters an appreciation for clinically driven research in an informal setting for providers at every level, including aspiring PIs like myself. In addition to informative clinical presentations, the CHEER network discusses the inner workings of “what to expect” when undertaking a clinical trial from start to finish. Experienced coordinators explain concepts such as IRB process, protocol design, and funding, while others provide insight on the “pitfalls” of clinical trials such as FDA audits. The conference instills feelings of appreciation for clinically oriented research and a greater desire to perform clinical research to enhance patient care for the next generation of patients. Lastly, it provides specific tools, connections, and resources that are needed to help facilitate successful clinical trials for coordinators and future PIs alike.