A Pivotal Year
John S. Rhee, MD, MPH, Research and Quality Improvement Coordinator This has been a pivotal year for Research and Quality Improvement as we continued to meet the specialty’s needs for quality knowledge products, expanded our efforts in implementation and dissemination of these products, revamped the CORE grants program, and researched plans for incorporating quality measurement development into the business unit to aid our members in meeting requirements for government and private payer quality programs and for Maintenance of Certification (MOC). The Patient Safety and Quality Improvement (PSQI) and Outcomes Research and Evidence Based Medicine (OREBM) Committees contribute greatly to these ongoing efforts. To improve implementation of clinical practice guidelines (CPGs) into practice, plain language summaries were developed on new guidelines. In addition, we partnered with an organization to provide pocket cards and apps for CPGs. Our dissemination and implementation efforts are not only focused on our specialty, but are geared also to other health professionals who treat patients with otolaryngology-head and neck surgery conditions. All of these activities require a tremendous volunteer effort and we now have a cohort of dedicated members who have developed expertise as chairs, co-chairs, panel members, methodologists, and implementation specialists for CPGs. We continue to be recognized within the national and international communities as a best practice organization in CPG development. Further details on our guidelines activity during the past year can be found in this issue of Bulletin. There will be an ongoing need for quality measures for our specialty in the foreseeable future. While changes based on the SGR are yet to be finalized, by all indications current quality programs requiring measure reporting at the individual physician level will continue to exist. However, for how long and what data exactly will be required is still not clear. Many private payers utilize programs such as Bridges to Excellence to create tiers of providers within their networks and these are also based on meeting specific quality measures. In addition, having quality measures for our specialty may assist physicians in meeting MOC requirements and are a natural outcome of the clinical practice guidelines process in terms of measuring adherence to guidelines. This past year, the Foundation has been in discussions with ABOto regarding the development of an otolaryngology-specific measure set utilizing existing measures in the Physician Quality Reporting System (PQRS) program. This would serve to reduce the reporting burden (less reporting is required if reporting on a measures group versus individual measures) and help our members get used to reporting to such programs. The latest data from CMS shows that about 36 percent of eligible otolaryngologists are reporting to PQRS. In the past, the Foundation has depended upon outside consortia, including the AMA-convened Physician Consortium for Performance Improvement® (PCPI), for the expertise to develop quality measures. This past year, a business plan outlining requirements for developing this expertise within the Research and Quality business unit was finalized and approved by the AAO-HNS/F Board. We continue to support a strong CORE grants program, which has provided numerous opportunities throughout the years for physician members to learn techniques for writing effective grant applications and to earn research grants. We are grateful to the many organizations that partner with us in these efforts. A review of the CORE grants program had not taken place since its inception at the Academy so this past year a board-level task force was appointed to review the program, to make sure we were still operating at optimal efficiency, and to identify areas for improvement. Based on the outcome of this work, some key changes were discussed with the funding organizations and changes will be incorporated for the 2015 grant cycle. A discussion of the CORE grants program, including this year’s grant recipients and a comprehensive list of the funding organizations, can be found on page 18. This marks the end of my term as the coordinator for Research and Quality Improvement and I want to formally welcome Lisa E. Ishii, MD, MHS, as the incoming coordinator. Lisa has the perfect background and skill set to help set the course as the Research and Quality Improvement business unit continues to evolve to meet the ongoing needs of our members.
John S. Rhee, MD, MPH, Research and
Quality Improvement Coordinator
This has been a pivotal year for Research and Quality Improvement as we continued to meet the specialty’s needs for quality knowledge products, expanded our efforts in implementation and dissemination of these products, revamped the CORE grants program, and researched plans for incorporating quality measurement development into the business unit to aid our members in meeting requirements for government and private payer quality programs and for Maintenance of Certification (MOC). The Patient Safety and Quality Improvement (PSQI) and Outcomes Research and Evidence Based Medicine (OREBM) Committees contribute greatly to these ongoing efforts.
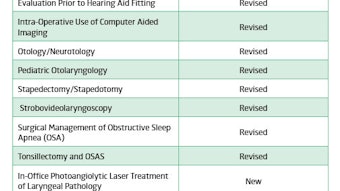
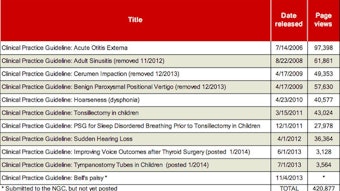
To improve implementation of clinical practice guidelines (CPGs) into practice, plain language summaries were developed on new guidelines. In addition, we partnered with an organization to provide pocket cards and apps for CPGs. Our dissemination and implementation efforts are not only focused on our specialty, but are geared also to other health professionals who treat patients with otolaryngology-head and neck surgery conditions. All of these activities require a tremendous volunteer effort and we now have a cohort of dedicated members who have developed expertise as chairs, co-chairs, panel members, methodologists, and implementation specialists for CPGs. We continue to be recognized within the national and international communities as a best practice organization in CPG development. Further details on our guidelines activity during the past year can be found in this issue of Bulletin.
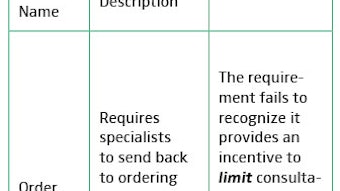
There will be an ongoing need for quality measures for our specialty in the foreseeable future. While changes based on the SGR are yet to be finalized, by all indications current quality programs requiring measure reporting at the individual physician level will continue to exist. However, for how long and what data exactly will be required is still not clear. Many private payers utilize programs such as Bridges to Excellence to create tiers of providers within their networks and these are also based on meeting specific quality measures. In addition, having quality measures for our specialty may assist physicians in meeting MOC requirements and are a natural outcome of the clinical practice guidelines process in terms of measuring adherence to guidelines. This past year, the Foundation has been in discussions with ABOto regarding the development of an otolaryngology-specific measure set utilizing existing measures in the Physician Quality Reporting System (PQRS) program. This would serve to reduce the reporting burden (less reporting is required if reporting on a measures group versus individual measures) and help our members get used to reporting to such programs. The latest data from CMS shows that about 36 percent of eligible otolaryngologists are reporting to PQRS. In the past, the Foundation has depended upon outside consortia, including the AMA-convened Physician Consortium for Performance Improvement® (PCPI), for the expertise to develop quality measures. This past year, a business plan outlining requirements for developing this expertise within the Research and Quality business unit was finalized and approved by the AAO-HNS/F Board.
We continue to support a strong CORE grants program, which has provided numerous opportunities throughout the years for physician members to learn techniques for writing effective grant applications and to earn research grants. We are grateful to the many organizations that partner with us in these efforts. A review of the CORE grants program had not taken place since its inception at the Academy so this past year a board-level task force was appointed to review the program, to make sure we were still operating at optimal efficiency, and to identify areas for improvement. Based on the outcome of this work, some key changes were discussed with the funding organizations and changes will be incorporated for the 2015 grant cycle. A discussion of the CORE grants program, including this year’s grant recipients and a comprehensive list of the funding organizations, can be found on page 18.
This marks the end of my term as the coordinator for Research and Quality Improvement and I want to formally welcome Lisa E. Ishii, MD, MHS, as the incoming coordinator. Lisa has the perfect background and skill set to help set the course as the Research and Quality Improvement business unit continues to evolve to meet the ongoing needs of our members.