Don’t Fear This Fungus: Allergic Fungal Rhinosinusitis
Like other subtypes of chronic rhinosinusitis, AFRS is managed with combined surgical and medical treatment.
Mason R. Krysinski, MD, and Philip G. Chen, MD
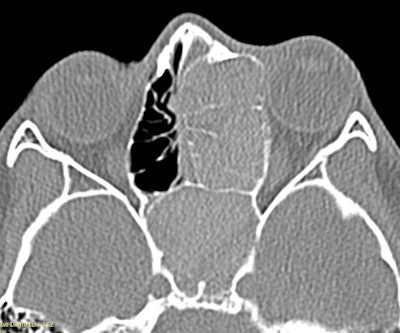 Figure 1. CT demonstrating the unilateral expansile disease process, which ultimately was determined as AFRS.
Figure 1. CT demonstrating the unilateral expansile disease process, which ultimately was determined as AFRS.
AFRS is a subtype of chronic rhinosinusitis with nasal polyps (CRSwNP) that accounts for approximately 5%-10% of chronic rhinosinusitis cases. It is characterized by a robust type 2 inflammatory profile, hypersensitivity to fungal antigens, and unique molecular pathways.1,2 Compared with other subtypes of CRSwNP, patients with AFRS tend to present younger and are more likely to present with unilateral findings. Further, AFRS is primarily seen in geographic regions with warm, humid climates.1,2,3
Clinically, AFRS often presents with substantial polyp burden and dense eosinophilic mucin with a “peanut butter” consistency (Figure 2A and B). CT can be helpful demonstrating classic findings of heterogeneous sinus opacification with areas of hyperattenuation and metallic-type “double densities” on soft tissue window (Figure 3).4 The disease process characteristically causes expansile changes to the paranasal sinuses, which can lead to expansion and erosion of the bony orbit, skull base, and facial skeleton (Figure 3).
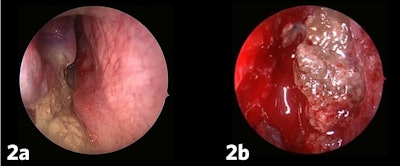 Figure 2a and b. AFRS often presents with substantial polyp burden and dense eosinophilic mucin with a “peanut butter” consistency.
Figure 2a and b. AFRS often presents with substantial polyp burden and dense eosinophilic mucin with a “peanut butter” consistency.
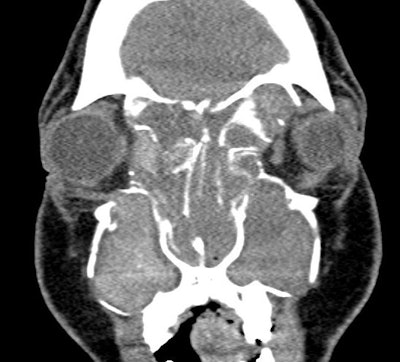 Figure 3. CT demonstrating classic findings of heterogeneous sinus opacification with areas of hyperattenuation and metallic-type “double densities” on soft tissue window causing expansile changes to the paranasal sinuses and leading to expansion and erosion of the bony orbit, skull base, and facial skeleton.
Figure 3. CT demonstrating classic findings of heterogeneous sinus opacification with areas of hyperattenuation and metallic-type “double densities” on soft tissue window causing expansile changes to the paranasal sinuses and leading to expansion and erosion of the bony orbit, skull base, and facial skeleton.
Although not necessary for diagnosis, surgeons may opt for MRI in cases of extensive orbital or skull base involvement. MRI demonstrates mucosal thickening with central hypointensity on T1- and T2-weighted sequences as well as a central signal void on T2-weighted scans due to metals produced by fungus.5 Bent and Kuhn’s initial diagnostic criteria for AFRS includes fungal type 1 hypersensitivity, nasal polyposis, characteristic CT findings, eosinophilic mucus without fungal invasion, and a positive fungal stain of sinonasal contents.6 Although Bent and Kuhn’s diagnostic criteria remain the most widely accepted diagnostic scheme, AFRS remains a clinical diagnosis, which is ultimately diagnosed at the time of surgery.1,2
Like other subtypes of chronic rhinosinusitis, AFRS is managed with combined surgical and medical treatment. Yet it is important to recognize the higher recurrence rate of AFRS compared with “garden variety” CRSwNP. Many recommend initial aggressive surgical management to remove fungal laden eosinophilic mucin followed by intermittent courses of postoperative oral and ongoing use of topical corticosteroids,2 which is our approach. Surgical management requires complete removal sinus bony partitions including concha bullosa, clearance of all fungal mucin, meticulous dissection, and wide osteoplasty to decrease risk of disease recurrence.1,2 Often, fungal debris and inspissated mucus are tenacious, and complete intraoperative removal requires tedious debridement and irrigation. Careful attention should be paid to the orbitocranial interface as expansile changes can erode overlying bone, resulting in a thin mucosal lining over periorbita and dura. Lastly, complete removal of mucin and fungal elements may require adjunct techniques such as maxillary mega-antrostomy, canine fossa puncture, or endoscopic modified Lothrop procedure. To date, isolated balloon sinus dilation lacks supportive evidence and balloon dilation is unlikely to widen already expanded ostia. Also, balloon-aided irrigation is typically insufficient to remove all fungal mucin, which is critical to minimize disease recurrence.
Medical management primarily consists of controlling inflammation. As a result, we regularly use perioperative oral corticosteroids, sinonasal irrigations, and topical corticosteroids. Given the high rate of recurrence of this disease, it is tempting to target fungus (Bipolaris, Curvularia, Alternaria, and Aspergillus are most common) as the culprit when edema and polyps start returning. To date, there is insufficient evidence to recommend standard antifungal use whether topical or systemic.1,2 This is especially important when considering the side effects of systemic therapy. Despite the amount of AFRS we encounter, we rarely add antifungal agents.
Type 1 hypersensitivity to fungus is a Bent and Kuhn diagnostic criterion, yet the role of immunotherapy remains controversial without a clear benefit in decreasing disease recurrence or improving quality of life.1,2 We work closely with our allergy colleagues to determine whether immunotherapy will benefit patients’ allergy symptoms, but it is not standard practice. Finally, we have anecdotally observed therapeutic benefits of biologic agents targeting type 2 inflammation for some patients suffering with recalcitrant AFRS. The patient in Figure 3 underwent three surgeries including an endoscopic modified Lothrop procedure, and the disease was not controlled until a biologic was started. Although promising, there is limited data regarding the role of biologics specifically in AFRS.
AFRS represents a distinct, clinically significant subtype of CRSwNP. While it shares features with other subtypes of chronic rhinosinusitis, it has important differences in pathophysiology, clinical presentation, and approaches to treatment. Continued investigation of the genetic, molecular, and immune pathways that drive the disease will aid further understanding of the disease process as the medical field continues to tailor treatments for our patients.
References
- Tyler MA, Luong AU. Current understanding of allergic fungal rhinosinusitis. World J Otorhinolaryngol Head Neck Surg. 2018 Nov 9;4(3):179-185. doi: 10.1016/j.wjorl.2018.08.003. PMID: 30506049; PMCID: PMC6251961.
- Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016 Feb;6(suppl 1):S22-209. doi: 10.1002/alr.21695. PMID: 26889651.
- Hoyt AE, Borish L, Gurrola J, Payne S. Allergic fungal rhinosinusitis. J Allergy Clin Immunol Pract. 2016 Jul-Aug;4(4):599-604. doi: 10.1016/j.jaip.2016.03.010. PMID: 27393774.
- Rowan NR, Janz TA, Schlosser RJ, Soler ZM. Radiographic nuances in allergic fungal rhinosinusitis. Am J Rhinol Allergy. 2019 May;33(3):310-316. doi: 10.1177/1945892419825695. Epub 2019 Jan 24. PMID: 30674195.
- Dykewicz MS, Rodrigues JM, Slavin RG. Allergic fungal rhinosinusitis. J Allergy Clin Immunol. 2018 Aug;142(2):341-351. doi: 10.1016/j.jaci.2018.06.023. PMID: 30080526.
- Bent JP 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994 Nov;111(5):580-8. doi: 10.1177/019459989411100508. PMID: 7970796.