OUT OF COMMITTEE | Outcomes Research and Evidence-Based Medicine
Targeted Therapy for Advanced Thyroid Cancer
Kevin J. Contrera, MD, MPh, Michael J. Brenner, MD (OREBM Committee Chair), Vikas Mehta, MD, Joseph Scharpf, MD, and Mark E. Zafereo, MD
The advent of targeted thyroid therapies represents one of the most significant advances in head and neck cancer in the past decade. Patients who were previously considered untreatable now have an opportunity for lifesaving interventions due to the development of several classes of new drugs targeting molecular mutations in thyroid malignancies. These drugs include multikinase inhibitors and tyrosine kinase inhibitors specific to mutations for fusions in BRAF, RET, TRK, and ALK. This brief review highlights novel therapeutics that have changed the landscape of advanced thyroid cancer treatment.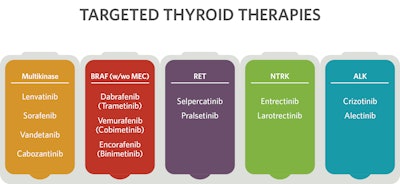
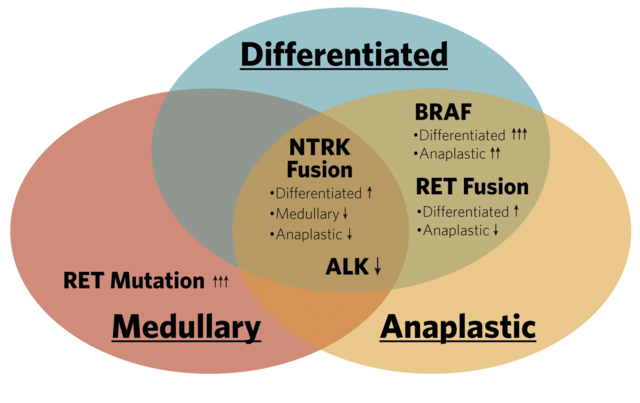
Frequency of Targetable Mutations/Fusions
Papillary and Follicular Thyroid Cancer
Surgery and radioactive iodine remain the standard of care for differentiated thyroid cancer, achieving cure in the vast majority of patients. However, some differentiated thyroid cancers demonstrate resistance to radioactive iodine. Lenvatinib and sorafenib are FDA-approved multikinase inhibitors for radioactive iodine-resistant differentiated thyroid cancer. Progression-free survivals were 18.3 and 10.8 months in lenvatinib (SELECT) and sorafenib (DECISION) phase III clinical trials, respectively.1,2 Based on these successes, targeted systemic therapy is now standard of care for radioactive iodine–resistant disease. These multikinase inhibitors and cabozantinib, recently approved as second-line therapy, have numerous targets, including VEGF, KIT, RET, and PDGFR.
Some differentiated thyroid cancers have somatic mutations such as BRAF or ALK, or gene fusions involving NTRK or RET that can be targeted by tyrosine kinase inhibitors, achieving durable locoregional and distant responses in select patients. BRAF V600E mutations are found in approximately half of papillary thyroid cancers. Dabrafenib and vemurafenib specifically target BRAF and may provide an alternative to multikinase inhibitors with fewer side effects. The median progression-free survival with vemurafenib was 18 months, comparable to lenvatinib, but the response rate of 39% is significantly less than 64% with lenvatinib.1,3 Somatic mutational and fusion testing for RET, NTRK, and ALK is recommended for advanced differentiated thyroid cancer that is negative for BRAF.4 Larotrectinib and entrectinib are FDA-approved for cancers with NTRK fusions, while RET-specific inhibitors are FDA-approved for differentiated thyroid cancers with RET fusions.
Follicular and Hurthle cell cancers are commonly associated with dysregulation of the RAS/RAF/MAPK and PI3K/AKT/mTOR pathways, although very rarely do they have mutations or fusions such as BRAF, RET, NTRK, or ALK, which are targetable with current specific inhibitors. Hurthle cell cancers may be associated with mTOR mutations, such that mTOR inhibitors (e.g., everolimus) may be effective. About 50% of follicular thyroid cancers harbor a RAS mutation, which is not specifically targetable with current inhibitors. Follicular and Hurthle cell cancers are therefore most commonly treated with multikinase inhibitors.
Medullary Thyroid Cancer
RET mutations are found in greater than 50% of sporadic medullary thyroid cancers and nearly all patients with hereditary disease. It is recommended that all patients with medullary thyroid cancer and their first-degree relatives be tested for germline RET (a simple blood test) to evaluate for MEN syndrome.4 For patients with advanced disease and RET-negative germline testing, somatic tumor testing is recommended to assess candidacy for upfront systemic therapy. RET can be targeted with RET-specific inhibitors, two of which have recently received FDA approval for the treatment of RET-altered thyroid cancers. Selpercatinib and pralsetinib have overall response rates of 73% and 71% for treatment-naïve patients, respectively.5,6 Surgery following RET-specific inhibitors is being studied in a clinical trial (NCT04759911). Cabozantinib and vandetanib are FDA-approved multikinase inhibitors for patients with or without RET mutations.
Anaplastic Thyroid Cancer
Approximately one-third of anaplastic thyroid cancers have a BRAF V600E mutation. Rapid BRAF testing is recommended for all patients diagnosed with anaplastic thyroid cancer, as inhibition of BRAF (and MEK, a resistance mechanism) has been associated with dramatic improvements in survival in these patients.4,7 Treatment with dabrafenib and trametinib achieved an overall response rate of 69% in a patient cohort with BRAF-V600-mutant locally advanced or metastatic anaplastic thyroid cancer.8 Surgery can be considered for resectable disease after treatment response (NCT04675710). Less commonly, anaplastic thyroid cancers can have targetable RET or NTRK fusions as well as ALK mutations. Mutations of p53, RAS, and TERT are frequent, but no therapeutic agents are currently available. Multikinase inhibitors (e.g., lenvatinib) have modest efficacy in patients with anaplastic thyroid cancer, and combining immunotherapy with targeted therapy can be considered for patients with PD-L1 positive tumors.
What Is to Come?
As our understanding of resistance mechanisms evolves, so too will the tools available for treating advanced thyroid cancers. While targeted therapies for thyroid cancers have dramatically improved treatment options, there remain many common mutations such as RAS, p53, and TERT, which are not specifically drug targetable. Combination therapies, including immunotherapy, are being investigated for their potential to delay or overcome resistance. Studies are ongoing looking into second-generation targeted medications (e.g., second-generation BRAF, RET, and NTRK inhibitors) and alternating between therapies. Numerous options are becoming available to mitigate side effects, which can include hypertension, proteinuria, and QT prolongation. The optimal timing of targeted treatment and the integration of standard modalities such as surgery and radioactive iodine continues to be studied. Clinical trials are evaluating the efficacy of targeted therapies in a neoadjuvant setting (e.g., NCT04759911).
Conclusion
Targeted therapies have redefined the therapeutic approach to advanced thyroid malignancies. Many questions are yet to be answered, but the advent of molecular therapies has revolutionized the field. Future advances will not only determine optimal regimens for targeted therapy but will also delineate the role of such approaches across the continuum of multidisciplinary therapy for thyroid malignancies.
The content of this article was developed in partnership with the American Academy of Otolaryngology−Head and Neck Surgery (AAO-HNS) Outcomes Research and Evidence-Based Medicine Committee. The content is provided by authors and does not reflect the official viewpoints of the AAO-HNS.
References
- Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372(7):621-630.
- Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384(9940):319-328.
- Brose MS, Cabanillas ME, Cohen EE, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17(9):1272-1282.
- Shonka DC, Jr., Ho A, Chintakuntlawar AV, et al. American Head and Neck Society Endocrine Surgery Section and International Thyroid Oncology Group consensus statement on mutational testing in thyroid cancer: defining advanced thyroid cancer and its targeted treatment. Head Neck. 2022.
- Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383(9):825-835.
- Subbiah V, Hu MI, Wirth LJ, et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): a multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021;9(8):491-501.
- Maniakas A, Dadu R, Busaidy NL, et al. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000-2019. JAMA Oncol. 2020;6(9):1397-1404.
- Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36(1):7-13.