Medicare Physician Fee Schedule (MPFS) 2014 Final Rule: What Does It Mean for You?
On November 27, 2013, the Centers for Medicare & Medicaid Services (CMS) posted the final rule for payments in the Medicare physician fee schedule (MPFS) for CY 2014. In addition to payment policy and payment rate updates, the MPFS addresses a number of quality initiatives. The Academy submitted comments to CMS on the final rule prior to the January 27, 2014, deadline (see www.bit.ly/CMSregs). Medicare Sustainable Growth Rate (SGR) The Medicare law includes the standard statutory formula that required (absent Congressional intervention) a 20.1 percent reduction to the CY 2014 conversion factor (CF) under the 2014 Medicare PFS. This would have resulted in a CY 2014 CF of $27.006, as compared to the CY 2013 CF of $34.0230. Congress acted, however, prior to the January 1 deadline to pass a three month patch that provided a .5% update to Medicare Payments through March 2014. Estimated Impact on Total Allowed Charges for ENT Services The overall impact of the CY 2014 final rule for otolaryngology-head and neck surgery is -2 percent. It is important to note that these estimates DO NOT INCLUDE the proposed reduction attributable to the SGR absent a Congressional fix prior to January 1, 2014. To fully understand the -2 percent impact to otolaryngology for CY 2014, members should be aware of the changes CMS is making to PE RVUs as it relates to the Medicare Economic Index formula. The MEI is an index that measures the price change of the inputs used to furnish physician services. CMS believes that the MEI is the best measure available of the relative weights of the three components in payments under the PFS—work, PE, and malpractice. CMS plans to hold the work RVUs constant and adjust the PE RVUs, the malpractice RVUs and the CF to produce the appropriate balance in RVUs among components and payments for CY 2014. The overall result of this policy change is that practitioners who furnish services with a higher proportion of PE RVUs (such as ENTs) will be most directly affected and should expect to see reductions across the board to PE RVUs for their services. Practice Expense Within the proposed rule, CMS proposed a major change to the methodology for setting practice expense RVUs for services under the PFS. They proposed to limit the office PE RVUs for individual codes so that the total office MPFS payment amount would not exceed the total combined amount Medicare pays for the same code in the facility (hospital or ASC) setting. As members recall, this policy would have significantly impacted 13 otolaryngology services by reducing their payment 6-60 percent for CY 2014, if finalized. Thanks to extensive advocacy efforts by the Academy, Congress, AMA, industry partners, and other specialty societies; CMS has chosen to retract this policy proposal within the 2014 Final rule. CMS expects to develop a revised proposal for using OPPS and ASC rates in developing PE RVUs that they will propose through future notice and comment rulemaking. They further clarify that they do not believe that the standard process for evaluating potentially misvalued codes, including the use of the AMA RUC, is an effective means of addressing these codes. Specifically, CMS believes the current review process for direct PE inputs only accommodates incomplete, small sample, and potentially biased or inaccurate resource input costs that may distort the resources used to develop non-facility (office) PE RVUs used in calculating PFS payment rates for individual services, and therefore, CMS plans to revisit this issue in future rulemaking. Potentially Misvalued Services Under the Fee Schedule CMS is also exploring new ways to broaden the process of identifying potentially misvalued codes. For the CY 2014 they proposed, and are now finalizing, a new process whereby they will solicit feedback from Medicare Contractor Medical Directors (CMDs) in developing a list of potentially misvalued codes. Improving Valuation of the Global Surgical Package In the CY 2013 proposed rule, CMS sought comments on methods of obtaining accurate and current data on E/M services furnished as part of a global surgical package. Commenters provided a variety of suggestions including comments from the AMA RUC noting that the hospital and discharge day management services included in the global period for many surgical procedures may have been inadvertently removed from the time file in 2007. CMS said in the CY 2013 final rule with comment period that they would review this file and, if appropriate, propose modifications to the physician time file in the CY 2014 PFS proposed rule. After extensive review, CMS agrees that the data were deleted from the time file due to an inadvertent error, as noted by the AMA RUC. Thus, they are replacing the missing post-operative hospital E/M visit information and time for the 117 codes that were identified by the AMA-RUC. Nine of the 117 CPT codes noted by the AMA RUC are provided by otolaryngology, and will be adjusted for CY 2014 accordingly. Physician Quality Reporting System (PQRS) For CY 2014, CMS finalized several changes to the PQRS program. Notable changes are outlined below: For 2014, CMS sets the incentive payment for satisfactory participation in PQRS at .05 percent of all Medicare Part B charges. For 2014 and beyond, CMS has set the penalty for unsatisfactory participation in PQRS at -2 percent of all Medicare Part B charges. Incentives are awarded in the performance year and penalties are applied to the reporting year, which is 2 years after the reporting year (e.g., 2014 reporting penalties would be applied to 2016 payments). CMS Changes to PQRS Measures and Measure Groups for CY 2014 Reporting CMS finalized 285 individual measures for inclusion in the 2014 PQRS program, including four of the Academy’s Sinusitis Measures for inclusion in 2014 and beyond. CMS did not finalize the proposed increase in the minimum number of measures in a measures group from four to six for CY 2014. They do state, however, that they plan to increase this minimum number in the future. As a result, CMS also does not finalize the proposed addition of measures to measures groups with less than six measures. CMS adds that it will work with the measure developers and owners of these measures groups to appropriately add measures to measures groups that only contain four measures. CMS Changes to PQRS Reporting Methods for CY 2014 CMS eliminated the option to report measure groups via claims for individual EPs in CY 2014. Individuals may now only report measure groups via registry. CMS reduced the percentage of patients EPs must report on using Registry reporting from the previous 80 percent to 50 percent for CY 2014 reporting. This is now consistent with the patient threshold requirements for reporting via claims. Changes to Individual PQRS Reporting in CY 2014 Reporting via Claims—CMS has increased the number of measures individual EPs must report on from the prior three to nine measures (across three quality domains, for 50 percent of beneficiaries) for CY 2014 reporting. EPs who report on less than nine measures will be subject to the Measure Applicability Validation (MAV) process. Reporting via Registry—CMS has increased the number of measures individual EPs must report on from the prior three to nine measures (across three quality domains, for 50 percent of beneficiaries) for CY 2014 reporting. EPs who report on less than nine measures will be subject to the MAV process. Reporting via Qualified Clinical Data Registry (NEW)—CMS finalized the new QCDR reporting option for individual reporting in CY 2014. CMS finalizes exceptions for individuals reporting via Claims and Registries for CY 2014 to avoid 2016 payment penalty. These EPs will not be eligible for the 2014 bonus payment, however. Changes to PQRS Group Reporting in CY 2014 CMS revised the deadline by which Group Practices choosing to report for PQRS via the Group Practice Reporting Option (GPRO) must self-nominate from the previous October 15 of the reporting year, to a new deadline of September 30 of the reporting year. CMS finalized a new group reporting option for groups of 25-99 EPs to report, via a CMS-certified survey vendor, on the CG-CAHPS survey measures. Groups selecting this reporting option will need to report using additional reporting methods in order to report on additional measures to meet the criteria for satisfactory reporting for CY 2014. CMS added the requirement for CY 2014 that groups of 25+ who wish to report the CG-CAHPS patient satisfaction survey measures must indicate their intent to do so when they register for the PQRS program. CMS also finalized a change to utilize a single website for Groups to self-nominate to use the GPRO reporting option as well as indicate they would like to report on CG-CAHPS measures for CY 2014. CMS added the requirement that groups of 100+ must report on all CG-CAHPS measures as well as the GPRO measures in the web interface. Physician Compare Website Under the ACA, CMS is required to develop a Physician Compare website with information on physicians enrolled in the Medicare program, as well as information on other EPs who participate in PQRS. In 2013, CMS released a redesigned Physician Compare website, which can be found at www.medicare.gov/physiciancompare. The primary source of administrative information on Physician Compare is from the Provider Enrollment, Chain, and Ownership System (PECOS), with the use of Medicare claims information to verify the information in PECOS. Providers must ensure information is up-to-date and accurate in the national PECOS database. To update information not found in PECOS, such as hospital affiliation and foreign language, providers should contact the Physician Compare team directly at physiciancompare@westat.com. Within the final rule, CMS finalized several new proposals for CY 2014 and beyond which are outlined in detail in the Academy summary of the 2014 final MPFS rule found at http://bit.ly/CMSregs. Members interested in more information on Physician Compare should also stay tuned for a Physician Compare specific article in the March edition of the Bulletin. Value Based Payment Modifier and Physician Feedback Reporting Program The VBM assesses both quality of care furnished and the cost of care under the MPFS. CMS has begun with a phase-in of the VBM in 2015, which will apply to all physicians by January 1, 2017. Implementation of the VBM is based on participation in PQRS. For CY 2013, the VBM applies to groups of physicians with 100 or more EPs. In 2014, CMS is expanding this to groups with 10+ EPs. For specifics on 2014 VBM requirements, additional information is available on the Academy website at http://bit.ly/entVBPM. CMS also issued regulations in several other areas affecting the otolaryngology community such as EHR Meaningful Use, e-Prescribing, ACOs, and more. We encourage members to view the more detailed summary on the finalized requirements for the programs highlighted above, at the Academy’s CMS Regulations and Comment letter page at http://bit.ly/CMSregs. Additionally, should you have any questions, please email the Health Policy staff at HealthPolicy@entnet.org.
On November 27, 2013, the Centers for Medicare & Medicaid Services (CMS) posted the final rule for payments in the Medicare physician fee schedule (MPFS) for CY 2014. In addition to payment policy and payment rate updates, the MPFS addresses a number of quality initiatives. The Academy submitted comments to CMS on the final rule prior to the January 27, 2014, deadline (see www.bit.ly/CMSregs).
Medicare Sustainable Growth Rate (SGR)
The Medicare law includes the standard statutory formula that required (absent Congressional intervention) a 20.1 percent reduction to the CY 2014 conversion factor (CF) under the 2014 Medicare PFS. This would have resulted in a CY 2014 CF of $27.006, as compared to the CY 2013 CF of $34.0230. Congress acted, however, prior to the January 1 deadline to pass a three month patch that provided a .5% update to Medicare Payments through March 2014.
Estimated Impact on Total Allowed Charges for ENT Services
The overall impact of the CY 2014 final rule for otolaryngology-head and neck surgery is -2 percent. It is important to note that these estimates DO NOT INCLUDE the proposed reduction attributable to the SGR absent a Congressional fix prior to January 1, 2014. To fully understand the -2 percent impact to otolaryngology for CY 2014, members should be aware of the changes CMS is making to PE RVUs as it relates to the Medicare Economic Index formula. The MEI is an index that measures the price change of the inputs used to furnish physician services. CMS believes that the MEI is the best measure available of the relative weights of the three components in payments under the PFS—work, PE, and malpractice. CMS plans to hold the work RVUs constant and adjust the PE RVUs, the malpractice RVUs and the CF to produce the appropriate balance in RVUs among components and payments for CY 2014. The overall result of this policy change is that practitioners who furnish services with a higher proportion of PE RVUs (such as ENTs) will be most directly affected and should expect to see reductions across the board to PE RVUs for their services.
Practice Expense
Within the proposed rule, CMS proposed a major change to the methodology for setting practice expense RVUs for services under the PFS. They proposed to limit the office PE RVUs for individual codes so that the total office MPFS payment amount would not exceed the total combined amount Medicare pays for the same code in the facility (hospital or ASC) setting. As members recall, this policy would have significantly impacted 13 otolaryngology services by reducing their payment 6-60 percent for CY 2014, if finalized.
Thanks to extensive advocacy efforts by the Academy, Congress, AMA, industry partners, and other specialty societies; CMS has chosen to retract this policy proposal within the 2014 Final rule. CMS expects to develop a revised proposal for using OPPS and ASC rates in developing PE RVUs that they will propose through future notice and comment rulemaking. They further clarify that they do not believe that the standard process for evaluating potentially misvalued codes, including the use of the AMA RUC, is an effective means of addressing these codes. Specifically, CMS believes the current review process for direct PE inputs only accommodates incomplete, small sample, and potentially biased or inaccurate resource input costs that may distort the resources used to develop non-facility (office) PE RVUs used in calculating PFS payment rates for individual services, and therefore, CMS plans to revisit this issue in future rulemaking.
Potentially Misvalued Services Under the Fee Schedule
CMS is also exploring new ways to broaden the process of identifying potentially misvalued codes. For the CY 2014 they proposed, and are now finalizing, a new process whereby they will solicit feedback from Medicare Contractor Medical Directors (CMDs) in developing a list of potentially misvalued codes.
Improving Valuation of the Global Surgical Package
In the CY 2013 proposed rule, CMS sought comments on methods of obtaining accurate and current data on E/M services furnished as part of a global surgical package. Commenters provided a variety of suggestions including comments from the AMA RUC noting that the hospital and discharge day management services included in the global period for many surgical procedures may have been inadvertently removed from the time file in 2007. CMS said in the CY 2013 final rule with comment period that they would review this file and, if appropriate, propose modifications to the physician time file in the CY 2014 PFS proposed rule. After extensive review, CMS agrees that the data were deleted from the time file due to an inadvertent error, as noted by the AMA RUC. Thus, they are replacing the missing post-operative hospital E/M visit information and time for the 117 codes that were identified by the AMA-RUC. Nine of the 117 CPT codes noted by the AMA RUC are provided by otolaryngology, and will be adjusted for CY 2014 accordingly.
Physician Quality Reporting System (PQRS)
For CY 2014, CMS finalized several changes to the PQRS program. Notable changes are outlined below: For 2014, CMS sets the incentive payment for satisfactory participation in PQRS at .05 percent of all Medicare Part B charges. For 2014 and beyond, CMS has set the penalty for unsatisfactory participation in PQRS at -2 percent of all Medicare Part B charges. Incentives are awarded in the performance year and penalties are applied to the reporting year, which is 2 years after the reporting year (e.g., 2014 reporting penalties would be applied to 2016 payments).
CMS Changes to PQRS Measures and Measure Groups for CY 2014 Reporting
CMS finalized 285 individual measures for inclusion in the 2014 PQRS program, including four of the Academy’s Sinusitis Measures for inclusion in 2014 and beyond.
CMS did not finalize the proposed increase in the minimum number of measures in a measures group from four to six for CY 2014. They do state, however, that they plan to increase this minimum number in the future. As a result, CMS also does not finalize the proposed addition of measures to measures groups with less than six measures. CMS adds that it will work with the measure developers and owners of these measures groups to appropriately add measures to measures groups that only contain four measures.
CMS Changes to PQRS Reporting Methods for CY 2014
CMS eliminated the option to report measure groups via claims for individual EPs in CY 2014. Individuals may now only report measure groups via registry.
CMS reduced the percentage of patients EPs must report on using Registry reporting from the previous 80 percent to 50 percent for CY 2014 reporting. This is now consistent with the patient threshold requirements for reporting via claims.
Changes to Individual PQRS Reporting in CY 2014
Reporting via Claims—CMS has increased the number of measures individual EPs must report on from the prior three to nine measures (across three quality domains, for 50 percent of beneficiaries) for CY 2014 reporting. EPs who report on less than nine measures will be subject to the Measure Applicability Validation (MAV) process.
Reporting via Registry—CMS has increased the number of measures individual EPs must report on from the prior three to nine measures (across three quality domains, for 50 percent of beneficiaries) for CY 2014 reporting. EPs who report on less than nine measures will be subject to the MAV process.
Reporting via Qualified Clinical Data Registry (NEW)—CMS finalized the new QCDR reporting option for individual reporting in CY 2014.
CMS finalizes exceptions for individuals reporting via Claims and Registries for CY 2014 to avoid 2016 payment penalty. These EPs will not be eligible for the 2014 bonus payment, however.
Changes to PQRS Group Reporting in CY 2014
CMS revised the deadline by which Group Practices choosing to report for PQRS via the Group Practice Reporting Option (GPRO) must self-nominate from the previous October 15 of the reporting year, to a new deadline of September 30 of the reporting year.
CMS finalized a new group reporting option for groups of 25-99 EPs to report, via a CMS-certified survey vendor, on the CG-CAHPS survey measures. Groups selecting this reporting option will need to report using additional reporting methods in order to report on additional measures to meet the criteria for satisfactory reporting for CY 2014.
CMS added the requirement for CY 2014 that groups of 25+ who wish to report the CG-CAHPS patient satisfaction survey measures must indicate their intent to do so when they register for the PQRS program. CMS also finalized a change to utilize a single website for Groups to self-nominate to use the GPRO reporting option as well as indicate they would like to report on CG-CAHPS measures for CY 2014.
CMS added the requirement that groups of 100+ must report on all CG-CAHPS measures as well as the GPRO measures in the web interface.
Physician Compare Website
Under the ACA, CMS is required to develop a Physician Compare website with information on physicians enrolled in the Medicare program, as well as information on other EPs who participate in PQRS. In 2013, CMS released a redesigned Physician Compare website, which can be found at www.medicare.gov/physiciancompare. The primary source of administrative information on Physician Compare is from the Provider Enrollment, Chain, and Ownership System (PECOS), with the use of Medicare claims information to verify the information in PECOS. Providers must ensure information is up-to-date and accurate in the national PECOS database. To update information not found in PECOS, such as hospital affiliation and foreign language, providers should contact the Physician Compare team directly at physiciancompare@westat.com.
Within the final rule, CMS finalized several new proposals for CY 2014 and beyond which are outlined in detail in the Academy summary of the 2014 final MPFS rule found at http://bit.ly/CMSregs. Members interested in more information on Physician Compare should also stay tuned for a Physician Compare specific article in the March edition of the Bulletin.
Value Based Payment Modifier and Physician Feedback Reporting Program
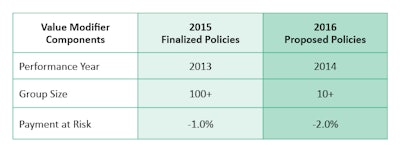
The VBM assesses both quality of care furnished and the cost of care under the MPFS. CMS has begun with a phase-in of the VBM in 2015, which will apply to all physicians by January 1, 2017. Implementation of the VBM is based on participation in PQRS. For CY 2013, the VBM applies to groups of physicians with 100 or more EPs. In 2014, CMS is expanding this to groups with 10+ EPs. For specifics on 2014 VBM requirements, additional information is available on the Academy website at http://bit.ly/entVBPM.
CMS also issued regulations in several other areas affecting the otolaryngology community such as EHR Meaningful Use, e-Prescribing, ACOs, and more. We encourage members to view the more detailed summary on the finalized requirements for the programs highlighted above, at the Academy’s CMS Regulations and Comment letter page at http://bit.ly/CMSregs. Additionally, should you have any questions, please email the Health Policy staff at HealthPolicy@entnet.org.