Acute Otitis Externa: Danger of Using Ototoxic Topical Drops
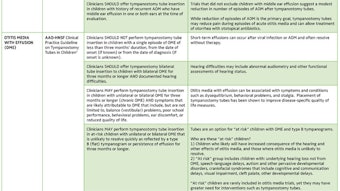
Adapted from Key Action Statement 7 of the CPG on Acute Otitis Externa Sujana S. Chandrasekhar, MD In the patient with acute otitis externa, if the tympanic membrane is known or suspected to be non-intact (including with the presence of a tympanostomy tube), topical drops that contain alcohol, have a low pH, or both should be AVOIDED because of pain and potential ototoxicity. Substances with ototoxic potential (e.g., aminoglycosides, alcohol) should NOT be utilized when the tympanic membrane is perforated and the middle ear space is open, because the risk of ototoxic injury outweighs the benefits compared to non-ototoxic antimicrobials with equal efficacy. The potential danger from administering an ototopical drop into the middle ear is the risk of its components reaching, and then crossing through, the round window membrane to affect the inner ear. Ototoxic antibiotics are used appropriately, for example, in Meniere’s disease, for their ability to cross the RWM and enter the cochlea and vestibule. The potential ototoxicity of such agents includes permanent SNHL and disequilibrium, and informed consent precedes that intervention. Clinical experience with topical ototoxic antibiotics in patients with tympanic membrane perforation suggests that hearing loss does not occur after a single short course of therapy; however, severe hearing loss has been observed after prolonged or repetitive administration of topical drops. There may be middle ear mucosal inflammation during the initial phase of AOE treatment in the case of the non-intact TM; as that swelling diminishes, the round window membrane actually becomes more accessible, and therefore the inner ear becomes potentially more susceptible to the deleterious effects of the ototoxic agents. Clinicians are advised to carefully evaluate the patient with AOE for presence of non-intact tympanic membrane by obtaining a thorough history and performing a comprehensive ear examination, including tympanometry as needed. Most tympanostomy tubes remain in the tympanic membrane for at least six to 12 months; therefore a patent tube should be assumed in that time frame, unless documented otherwise. Tubes may, of course, remain functional for three years or longer. Individuals who taste medicines placed into their ear, or who can expel air out of their ear canal by pinched nose blowing, can be assumed to have a perforation. The only topical antimicrobials approved by the FDA (December 2005) for middle ear use are quinolone drops. Additionally, there is an explicit warning by the manufacturer that neomycin/polymyxin B/hydrocortisone should NOT be used with a non-intact tympanic membrane, and that warning cites explicitly the risk of permanent SNHL with its use. It is reiterated, therefore: when treating a patient with acute otitis externa, obtain a focused history and perform an otological examination that will, in addition to defining the AOE, determine the status of the tympanic membrane. If you know or suspect that there is a TM perforation or PE tube, do NOT use ototoxic eardrops. The evidence supporting this is level D with a moderate level of confidence in the evidence.
Adapted from Key Action Statement 7 of the CPG on Acute Otitis Externa
Sujana S. Chandrasekhar, MD
In the patient with acute otitis externa, if the tympanic membrane is known or suspected to be non-intact (including with the presence of a tympanostomy tube), topical drops that contain alcohol, have a low pH, or both should be AVOIDED because of pain and potential ototoxicity. Substances with ototoxic potential (e.g., aminoglycosides, alcohol) should NOT be utilized when the tympanic membrane is perforated and the middle ear space is open, because the risk of ototoxic injury outweighs the benefits compared to non-ototoxic antimicrobials with equal efficacy.
The potential danger from administering an ototopical drop into the middle ear is the risk of its components reaching, and then crossing through, the round window membrane to affect the inner ear. Ototoxic antibiotics are used appropriately, for example, in Meniere’s disease, for their ability to cross the RWM and enter the cochlea and vestibule. The potential ototoxicity of such agents includes permanent SNHL and disequilibrium, and informed consent precedes that intervention.
Clinical experience with topical ototoxic antibiotics in patients with tympanic membrane perforation suggests that hearing loss does not occur after a single short course of therapy; however, severe hearing loss has been observed after prolonged or repetitive administration of topical drops. There may be middle ear mucosal inflammation during the initial phase of AOE treatment in the case of the non-intact TM; as that swelling diminishes, the round window membrane actually becomes more accessible, and therefore the inner ear becomes potentially more susceptible to the deleterious effects of the ototoxic agents.
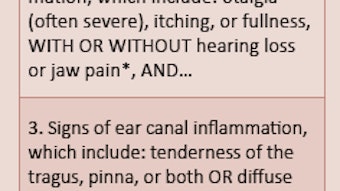
Clinicians are advised to carefully evaluate the patient with AOE for presence of non-intact tympanic membrane by obtaining a thorough history and performing a comprehensive ear examination, including tympanometry as needed. Most tympanostomy tubes remain in the tympanic membrane for at least six to 12 months; therefore a patent tube should be assumed in that time frame, unless documented otherwise. Tubes may, of course, remain functional for three years or longer. Individuals who taste medicines placed into their ear, or who can expel air out of their ear canal by pinched nose blowing, can be assumed to have a perforation.
The only topical antimicrobials approved by the FDA (December 2005) for middle ear use are quinolone drops. Additionally, there is an explicit warning by the manufacturer that neomycin/polymyxin B/hydrocortisone should NOT be used with a non-intact tympanic membrane, and that warning cites explicitly the risk of permanent SNHL with its use.
It is reiterated, therefore: when treating a patient with acute otitis externa, obtain a focused history and perform an otological examination that will, in addition to defining the AOE, determine the status of the tympanic membrane. If you know or suspect that there is a TM perforation or PE tube, do NOT use ototoxic eardrops.
The evidence supporting this is level D with a moderate level of confidence in the evidence.