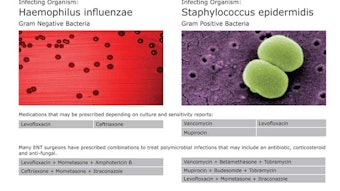
Another Survey?
Rahul K. Shah, MD George Washington University School of Medicine, Children’s National Medical Center, Washington, DC The Patient Safety and Quality Improvement (PSQI) Committee, which I co-chair with David W. Roberson, MD, has a broad charge—essentially to help ensure that Academy members are kept abreast of, and are leading, efforts toward improving the safety and quality of the care that is delivered to our patients. In the past decade, there have been tremendous efforts to understand the scope of the problem vis à vis adverse events, near misses, and medical/surgical error. These efforts have been led by myriad stakeholders, each with interests central to their strategy—non-otolaryngologist physicians, hospitals, insurance companies, medical liability insurers, and even the government. As a vulnerability of most research methodology, the outcomes can be somewhat predicted or manipulated based on the techniques employed to study an issue. PSQI efforts are not immune to this bias. To approach studying these issues, we can employ various research methodologies. Of course, the most robust research could be constructed to test a hypothesis regarding PSQI. Whatever confidence is gained by proper study design needs to be balanced with the resources necessary to do such a study. This would certainly be credible data, however, resources are not limitless and such a methodology may take significant time and resources and leave certain aspects of PSQI unstudied or unreported. A hallmark of most quality improvement projects is the Plan-Do-Study-Act (PDSA) cycle of iterative quality improvement. Perhaps we would be able to conduct a tremendous amount of PSQI work using the PDSA cycle and postulating strategies to reduce harm and errors. However, the ability to extrapolate these findings and the lack of rigor precludes using the PDSA as a sole source of PSQI work. The use of survey methodology has emerged as the workhorse for the PSQI committee. With the high number of responses and the excellent qualitative nature of the responses by Academy members, we have learned a tremendous amount about PSQI using surveys. The survey methodology allows us to cast a wide net and then lets the data guide us in future areas that need to be targeted. For example, we did not realize the scope of the problem with concentrated epinephrine in otolaryngology surgery or the role of inverted computed tomography scans in sinus surgery until they were highlighted qualitatively in free-text surveys. The survey tool is, in our opinion, an excellent mechanism to help us hone in on specific zones of risk in a discipline or with a procedure. Once we have broad data from the surveys, we then develop more robust research methodologies to take a deep, more quantitative dive into the data. Of course, it is imperative to acknowledge the Academy membership and your willingness to spend 10 minutes to answer myriad surveys. If you feel that surveys have become quite numerous as of late—you are correct. The landscape in the PSQI arena is constantly changing and being driven by many stakeholders. As such, the PSQI committee believes that the best way to understand the problem as it affects our members is via data and studies. It is imperative that we as otolaryngologists construct the surveys to ensure that the questions are appropriate for our patients and our practice types and furthermore to ensure integrity in reporting the results and understanding the context of the findings from the studies. If our specialty continues to produce PSQI-related work products and continues to lead the way, then perhaps other stakeholders will take our work and use it rather than relying on their own “research” into PSQI issues in our specialty. Ideally, it would be great to partner with other stakeholders to attempt to identify solutions for PSQI-related issues. Nevertheless, we should be leading the majority of the work in the PSQI as it directly pertains to our practices and we are the content experts. It is hard to know what the PSQI landscape will bring in the coming years, but I can be certain that the PSQI committee will think of techniques so that we can continuously provide a forum for members’ viewpoints and issues to be collated and properly disseminated. We can be certain that there will probably be another survey—soon! We encourage members to write us with any topic of interest and we will try to research and discuss the issue. Members’ names are published only after they have been contacted directly by Academy staff and have given consent to the use of their names. Please email the Academy at qualityimprovement@entnet.org to engage us in a patient safety and quality discussion that is pertinent to your practice.
Rahul K. Shah, MD
George Washington University School of Medicine, Children’s National Medical Center, Washington, DC
The Patient Safety and Quality Improvement (PSQI) Committee, which I co-chair with David W. Roberson, MD, has a broad charge—essentially to help ensure that Academy members are kept abreast of, and are leading, efforts toward improving the safety and quality of the care that is delivered to our patients.
In the past decade, there have been tremendous efforts to understand the scope of the problem vis à vis adverse events, near misses, and medical/surgical error. These efforts have been led by myriad stakeholders, each with interests central to their strategy—non-otolaryngologist physicians, hospitals, insurance companies, medical liability insurers, and even the government. As a vulnerability of most research methodology, the outcomes can be somewhat predicted or manipulated based on the techniques employed to study an issue.
PSQI efforts are not immune to this bias. To approach studying these issues, we can employ various research methodologies. Of course, the most robust research could be constructed to test a hypothesis regarding PSQI. Whatever confidence is gained by proper study design needs to be balanced with the resources necessary to do such a study. This would certainly be credible data, however, resources are not limitless and such a methodology may take significant time and resources and leave certain aspects of PSQI unstudied or unreported.
A hallmark of most quality improvement projects is the Plan-Do-Study-Act (PDSA) cycle of iterative quality improvement. Perhaps we would be able to conduct a tremendous amount of PSQI work using the PDSA cycle and postulating strategies to reduce harm and errors. However, the ability to extrapolate these findings and the lack of rigor precludes using the PDSA as a sole source of PSQI work.
The use of survey methodology has emerged as the workhorse for the PSQI committee. With the high number of responses and the excellent qualitative nature of the responses by Academy members, we have learned a tremendous amount about PSQI using surveys. The survey methodology allows us to cast a wide net and then lets the data guide us in future areas that need to be targeted. For example, we did not realize the scope of the problem with concentrated epinephrine in otolaryngology surgery or the role of inverted computed tomography scans in sinus surgery until they were highlighted qualitatively in free-text surveys.
The survey tool is, in our opinion, an excellent mechanism to help us hone in on specific zones of risk in a discipline or with a procedure. Once we have broad data from the surveys, we then develop more robust research methodologies to take a deep, more quantitative dive into the data.
Of course, it is imperative to acknowledge the Academy membership and your willingness to spend 10 minutes to answer myriad surveys. If you feel that surveys have become quite numerous as of late—you are correct. The landscape in the PSQI arena is constantly changing and being driven by many stakeholders. As such, the PSQI committee believes that the best way to understand the problem as it affects our members is via data and studies.
It is imperative that we as otolaryngologists construct the surveys to ensure that the questions are appropriate for our patients and our practice types and furthermore to ensure integrity in reporting the results and understanding the context of the findings from the studies. If our specialty continues to produce PSQI-related work products and continues to lead the way, then perhaps other stakeholders will take our work and use it rather than relying on their own “research” into PSQI issues in our specialty. Ideally, it would be great to partner with other stakeholders to attempt to identify solutions for PSQI-related issues. Nevertheless, we should be leading the majority of the work in the PSQI as it directly pertains to our practices and we are the content experts.
It is hard to know what the PSQI landscape will bring in the coming years, but I can be certain that the PSQI committee will think of techniques so that we can continuously provide a forum for members’ viewpoints and issues to be collated and properly disseminated. We can be certain that there will probably be another survey—soon!
We encourage members to write us with any topic of interest and we will try to research and discuss the issue. Members’ names are published only after they have been contacted directly by Academy staff and have given consent to the use of their names. Please email the Academy at qualityimprovement@entnet.org to engage us in a patient safety and quality discussion that is pertinent to your practice.