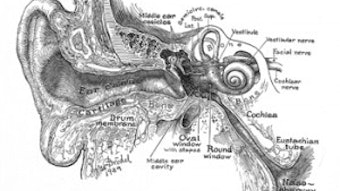
Kids ENT Health and the Academy: Then and Now
“Many things can wait; the child cannot. Now is the time his bones are being formed, his mind is being developed. To him we cannot say tomorrow; his name is today.” — Gabriela Mistral (1889-1957), the first Latin American woman to win a Nobel Prize in Literature For a decade now, this Academy has been using the month of February to promote children’s ear, nose, throat, head, and neck health. The official name of the observation is Kids E.N.T. Health Month. In 2003 we asked “Why Kids E. N. T. Health?” Children suffer from maladies and issues of the ears, nose, and throat and related structures more often than any other part of the body. Providing parents, caregivers, referring physicians, and allied health professionals the most up-to-date information on the diagnosis and treatment of childhood ear, nose, and throat disorders is crucial to the health and wellness of America’s children. Research has shown that sensory development [in a safe environment] is a necessity for children to fully explore their environment and enjoy an uninhibited learning experience. Left untreated or improperly treated, illnesses involving the ears, nose, and throat, can impede proper development of the senses, leading to learning disabilities. Since then, the campaign’s focus and the Academy’s work not only has included clinical issues affecting children, but also has dealt with social issues such as supporting legislation for newborn hearing screening, pointing out the dangers of second-hand smoke, environmental noise pollution, and the perils of ingesting foreign objects. Past campaigns reached many communities. Between 2003 and 2007, the campaign attracted grassroots media, and garnered millions of impressions nationwide including two syndicated publications: Health Behavior News Service [a tool for health reports]: “Non-steroidal anti-inflammatory drugs and preoperative bleeding in tonsillectomy;” and “Administration of antibiotics to children aged five and younger if green nasal discharge is present,” in Parenting Magazine with more than two million readers. Also of note was the NAPS Extraordinary Achievement Award in 2005 for 753 radio airings for “Treating Allergies Seriously” from this syndication service, placing it in the top one-quarter of one percent of all its releases, including most Fortune 500 companies and more than 100 associations, the Top 12 public relations firms, and 1,600 accounts. Much has changed since 2007, including the spread of information technology, which has allowed healthcare providers to collect more information more quickly and be able to analyze treatment options and see their outcomes.The AAO-HNS/F has been making the most of this through Research and Quality Improvement (R and QI) activities. The R and QI activities include the garnering of systematic evidence-based data to develop guidelines and consensus statements for Members to use in improving care. Both clinical and educational committees of the Academy and Foundation participate in guideline development, as do consumer groups. The theme for this year’s outreach campaign is “Kids E.N.T. Health: Safety.”David E. Tunkel, MD, Pediatric Otolaryngology Committee chair, withIan N. Jacobs, MD, of that committee, contributes information on two children’s health safety issues. Wendy B. Stern, MD, chair of the Media and Public Relations Committee, gives us a wonderful overview of what it means to champion children’s health issues in our communities today. In addition, our Foundation in partnership with the American Society of Pediatric Otolaryngology, is offering a 10-webinar series on vital care topics outlined here. Of course, there are still many issues surrounding children’s E.N.T. Health to address. In 2012, 15 states still did not require newborn hearing screening. With information traveling so quickly, we have a wonderful opportunity to make use of emerging networks of health bloggers and mobile bloggers to extend the reach of our own grassroots educational efforts.Academy Helps Members to Extend Care Unique Academy campaign resources, available to members through the Academy’s website, can be used to begin the campaign in your community. Here you will find a list of resources at www.entnet.org/AboutUs/KidsENT.cfm. The communications staff is also available for consultation and support. Our government affairs and regulatory advocacy teams are constantly promoting health and access-to-care issues with legislators and regulatory agencies. As we think of educating our communities this month, also be aware of the opportunities to work with patients in discussing treatments that are right for them. As you know, the AAO-HNSF has joined 32 other medical specialty societies and the American Board of Internal Medicine (ABIM) Foundation (34 total) in the Choosing Wisely® campaign to promote wise choices between physicians and patients to improve healthcare outcomes, provide patient-centered care that avoids unnecessary and even harmful interventions, and reduce the rapidly-expanding costs of the healthcare system.
“Many things can wait; the child cannot. Now is the time his bones are being formed, his mind is being developed. To him we cannot say tomorrow; his name is today.” — Gabriela Mistral (1889-1957), the first Latin American woman to win a Nobel Prize in Literature
For a decade now, this Academy has been using the month of February to promote children’s ear, nose, throat, head, and neck health. The official name of the observation is Kids E.N.T. Health Month.
In 2003 we asked “Why Kids E. N. T. Health?” Children suffer from maladies and issues of the ears, nose, and throat and related structures more often than any other part of the body. Providing parents, caregivers, referring physicians, and allied health professionals the most up-to-date information on the diagnosis and treatment of childhood ear, nose, and throat disorders is crucial to the health and wellness of America’s children.
Research has shown that sensory development [in a safe environment] is a necessity for children to fully explore their environment and enjoy an uninhibited learning experience. Left untreated or improperly treated, illnesses involving the ears, nose, and throat, can impede proper development of the senses, leading to learning disabilities.
Since then, the campaign’s focus and the Academy’s work not only has included clinical issues affecting children, but also has dealt with social issues such as supporting legislation for newborn hearing screening, pointing out the dangers of second-hand smoke, environmental noise pollution, and the perils of ingesting foreign objects.
Past campaigns reached many communities. Between 2003 and 2007, the campaign attracted grassroots media, and garnered millions of impressions nationwide including two syndicated publications: Health Behavior News Service [a tool for health reports]: “Non-steroidal anti-inflammatory drugs and preoperative bleeding in tonsillectomy;” and “Administration of antibiotics to children aged five and younger if green nasal discharge is present,” in Parenting Magazine with more than two million readers. Also of note was the NAPS Extraordinary Achievement Award in 2005 for 753 radio airings for “Treating Allergies Seriously” from this syndication service, placing it in the top one-quarter of one percent of all its releases, including most Fortune 500 companies and more than 100 associations, the Top 12 public relations firms, and 1,600 accounts.
The theme for this year’s outreach campaign is “Kids E.N.T. Health: Safety.”David E. Tunkel, MD, Pediatric Otolaryngology Committee chair, withIan N. Jacobs, MD, of that committee, contributes information on two children’s health safety issues. Wendy B. Stern, MD, chair of the Media and Public Relations Committee, gives us a wonderful overview of what it means to champion children’s health issues in our communities today. In addition, our Foundation in partnership with the American Society of Pediatric Otolaryngology, is offering a 10-webinar series on vital care topics outlined here.
Unique Academy campaign resources, available to members through the Academy’s website, can be used to begin the campaign in your community. Here you will find a list of resources at www.entnet.org/AboutUs/KidsENT.cfm.
The communications staff is also available for consultation and support. Our government affairs and regulatory advocacy teams are constantly promoting health and access-to-care issues with legislators and regulatory agencies.
As we think of educating our communities this month, also be aware of the opportunities to work with patients in discussing treatments that are right for them. As you know, the AAO-HNSF has joined 32 other medical specialty societies and the American Board of Internal Medicine (ABIM) Foundation (34 total) in the Choosing Wisely® campaign to promote wise choices between physicians and patients to improve healthcare outcomes, provide patient-centered care that avoids unnecessary and even harmful interventions, and reduce the rapidly-expanding costs of the healthcare system.