Thyroid Ultrasound and Molecular Testing: Knowing the Nodule
Understanding and interpreting molecular tests for thyroid nodules.
Robert L. Witt, MD
The American Thyroid Association (ATA) guidelines and TIRADS are the most widely used risk stratification systems in the United States. High-risk ultrasound features (hypoechogenic nodule, microcalcifications, irregular margins, extrathyroidal spread, taller than wide on transvers ultrasound view) can influence the decision to add molecular testing. For example, a thyroid nodule with high-risk ultrasound features may prompt diagnostic surgery rather than molecular testing in selected cases.
There are currently three commercially available thyroid molecular tests in the United States: the Genomic Sequencing Classifier (Afirma), ThyroSeq v3 (CBL Path), and ThyGeNext + ThyraMIRv2 (Interpace). Generally, molecular testing is deployed for indeterminate thyroid nodules that are follicular lesion of unknown significance or atypia of unknown significance (Bethesda grade 3) and follicular neoplasm or Hürthle cell neoplasm (Bethesda grade 4).
 FLUS/FN Algorithm: Afirma GSC-Xpression Atlas or NGSv3 or ThyGeNext/ThyraMIR
FLUS/FN Algorithm: Afirma GSC-Xpression Atlas or NGSv3 or ThyGeNext/ThyraMIR
Indeterminate thyroid cytology constitutes about 20%-30% of thyroid nodules, of which approximately 80% are benign post-surgery. The principal breakthrough of molecular testing has been to reduce the percentage of surgical procedures on benign nodules in selected cases of indeterminate cytology with benign molecular testing. Molecular testing for indeterminate thyroid nodules should either improve sensitivity analysis and reduce surgery on benign nodules or improve specificity analysis and reduce the number of completion thyroidectomies on malignant nodules.
The surgeon should understand that molecular tests are not intended to replace clinical evaluation. When considering molecular testing the patient should be advised of potential benefits and limitations of molecular testing and possible uncertainties in the therapeutic and long-term clinical implications of results.
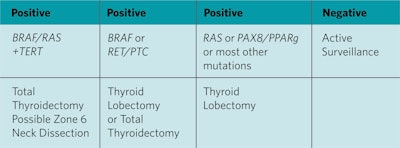
Genetics help to interpret the usefulness of molecular testing. Seventy-five percent of papillary thyroid cancers (PTC) are either BRAF (45%), RET/PTC (15%), or RAS (15%). The BRAFV600E (BRAF) point mutation is the most common mutation in PTC. BRAF is nearly 100% specific for PTC. The surgeon and patient can determine a therapeutically optimal operation of lobectomy or total thyroidectomy with near certainty that the final pathologic diagnosis will be PTC. Although the BRAF mutation has been associated with extrathyroidal spread and lymph node metastases, most tumors do not behave aggressively. A positive BRAF mutation is an indication for surgery, however, it is not currently a guide to the extent of surgery, central compartment neck dissection or radioactive iodine. Thyroid lobectomy or total thyroidectomy and the risks of each can be discussed with the patient as therapeutic options.
The RAS point mutation is the most common mutation in an indeterminate thyroid nodule. Unfortunately, it is not highly specific for malignancy. RAS+ nodules are often either benign, low-grade follicular variant of papillary thyroid carcinoma (FVPTC), or noninvasive thyroid neoplasm with papillary-like nuclear features (NIFTP). NIFTP has limited malignant potential and is best managed by thyroid lobectomy. There is a growing role for limited surgery (thyroid lobectomy) for a cytologically indeterminate RAS+ thyroid nodule as it will provide adequate treatment for most low-risk malignancies, NIFTP, or benign nodules without exposing the patient to the risks of total thyroidectomy. The classification of NIFTP has lowered the percentage of RAS+ mutations that are malignant, currently ranging from 40% to 60%.
The most common chromosomal rearrangement mutation is RET/PTC. It is nearly 100% specific for PTC. Similar to the BRAF mutation, a therapeutically optimal operation of lobectomy or total thyroidectomy can be determined. BRAF and RET/PTC mutations “rule in” PTC with near certainty.
The TERT mutation is not common; however, it does have prognostic significance and is associated with an aggressive thyroid malignancy. Treatment for a nodule with a TERT mutation is total thyroidectomy and possible central compartment dissection. The TERT mutation has an occasional false positive for malignancy.
The first thyroid cancer “rule out” molecular test was the gene expression classifier, now called the genomic sequencing classifier (GSC), an RNA-based next generation sequencer. In contrast to the high specificity and high positive predictive value (PPV) of BRAF and RET/PTC, the GSC has greater sensitivity and negative predictive value (NPV). The result of the GSC is reported as either benign or suspicious. A benign result for a Bethesda 3 or 4 cytologically indeterminate thyroid nodule has a 95% NPV equivalent to a cytologically benign Bethesda 2 thyroid nodule.3 A patient without a high-risk history or exam and a thyroid nodule with no high-risk ultrasound features could potentially be offered active surveillance in selected cases rather than diagnostic thyroid lobectomy. The benign call rate for patients with indeterminate thyroid nodules is about 50%-60% and these patients can potentially be spared surgery.The PPV of the GSC is about 50%. The current iteration of the GSC includes BRAF and RET/PTC rule in mutation tests allowing definitive surgery in these cases. In addition, the Afirma Xpression Atlas can be selectively ordered. It uses 593 genes for identification of other mutations. The vast majority of mutations other than the BRAF and RET/PTC are not specific for thyroid cancer, and diagnostic lobectomy is often the optimal procedure for most other mutations. Completion thyroid lobectomy may not be required for malignancy if the nodule is less than 4 cm per ATA guidelines in selected nodules.
ThyroSeqv3 is a DNA and mRNA next generation sequencing and expression test. The test provides a positive or negative result along with any detected mutation. ThyroSeqv3 has an NPV equivalent to the GSC. It has a PPV of 66% and a benign call rate of 60%.4 This test is marketed as a rule in and rule out test; however, unless a mutation is positive for BRAF or RET/PTC, the test is not specific for thyroid malignancy, and diagnostic thyroid lobectomy is often the optimal procedure for a mutation that is not BRAF or RET/PTC.
ThyGenNext/ThyraMIRv2 is a DNA and mRNA next generation sequencing test with PCR expression for 11 miRNAs. miRNAs are small, noncoding RNAs that bind to messenger RNA and regulate expression of genes involved in human cancers (posttranscriptional). ThyGenNext/ThyraMIRv2 has an NPV equivalent to the GSC and ThyroSeqv3. A PPV of 75% has been reported.5 ThyGenNext is a panel of point mutations and fusions (chromosomal rearrangements) overlapping what is offered in the Afirma Xpression and the ThyroSeqv3 tests. If either no mutation or a weak driver mutation like RAS is found on the ThyGenNext mutation panel, the miRNA panel is ordered reflexively. If no positive miRNA is found, active surveillance can be considered in selected cases, including RAS+ mutation or other weak driver mutation (excluding BRAF and RET/PTC). However, further validation for active surveillance of a RAS+ nodule or other weak driver mutation with no positive miRNA should be considered.
The wheelhouse for molecular testing is the rule out test for the cytologically indeterminate thyroid nodule. All three commercially available thyroid molecular tests provide a similar NPV for indeterminate thyroid nodules (Bethesda grades 3 and 4) with a benign result. The NPV is equivalent to the NPV for a cytologically benign, Bethesda grade 2 thyroid nodule; however, it will drop if a given institution has a prevalence of malignancy in indeterminate thyroid nodules that exceeds 30%, which is fortunately not common.
All three molecular tests improve the PPV (rule in) for indeterminate thyroid nodules over cytology alone (from approximately 20%-30% to 40%-75%); however, none come close to providing a rule in for thyroid cancer equivalent to a Bethesda grade 6 (98% PPV for cytologically malignant).
There are several concerns with molecular testing, including a lack of a true gold standard in the subsequent validation studies where very few of the GSC, ThyroSeqv3, or ThyGenNext/ThyraMIRv2 benign cases have histologic correlation, that is, go on to diagnostic surgery. Future work needs to concentrate on long-term prospective validation of false positives and negatives, cost-effectiveness studies, and head-to-head comparisons. Furthermore, real-world studies as opposed to industry-sponsored studies suggest lower PPV and NPV, and this needs further investigation. Hürthle cell neoplasms and their accuracy in molecular testing need further study and refinement as they exhibit various molecular alterations without consistent association to histologically benign or malignant cases.
Do molecular alteration tests for indeterminate thyroid nodules improve sensitivity analysis and NPV? The answer is yes. Can surgery be avoided in selected cases with molecular tests? Yes, it can with careful follow-up as there is limited testing against a true gold standard.
Do molecular alteration tests for indeterminate thyroid nodules improve specificity analysis and PPV? The answer is somewhat. Do molecular alteration tests reduce the number of completion thyroidectomies? The answer is probably yes with an assist from the ATA guidelines sanctioning lobectomy or total thyroidectomy for nodules from 1 to 4 cm.