Driving Quality in Otolaryngology through CPGs and ECSs
Academy members can propose topics for clinical practice guidelines and expert consensus statements, serve on development panels, and review draft documents as peer reviewers or during public comment.
David E. Tunkel, MD, Guideline Task Force Chair
As otolaryngology–head and neck surgeons, we must be involved in developing and implementing clinically valid quality measures and take the lead in defining what “quality care” means for our specialty. The American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) Guideline Task Force (GTF) continues to provide guidance for the development and implementation of two of the Foundation’s main quality products: Clinical Practice Guidelines (CPGs) and Expert Consensus Statements (ECSs). Here, I provide updates on recent and upcoming guidelines and statements and explain pathways for getting involved in development and review.
Recently Developed and In-Progress Quality Products
Earlier this year, CPGs Age-Related Hearing Loss and Immunotherapy for Inhalant Allergy were developed by panels comprising representatives of stakeholders from multiple disciplines and published in the March and May issues, respectively, of Otolaryngology-Head and Neck Surgery. During the last two years, ECSs Dysphagia in Head and Neck Cancer Patients and Pediatric Persistent Obstructive Sleep Apnea After Adenotonsillectomy were also developed by panels of experts in these fields.
By the time you read this article, a single-specialty CPG on Surgical Management of Rhinosinusitis, developed by a panel chaired by Jennifer Shin, MD, will be well into its multiple stages of review and editing. Concurrently, a second update to the CPG Adult Sinusitis (last updated in 2015) is underway. Spencer C. Payne, MD, is serving as Chair.
How CPGs and ECSs Are Proposed and Evaluated
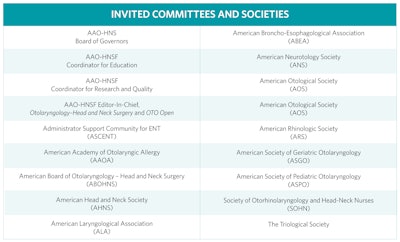
The GTF assembles representatives from committees and societies inside and outside the AAO-HNS/F. (See “Invited Committees and Societies” below.) This group meets twice yearly (in-person and virtually) to discuss the guideline development process, as well as to share the needs and views of the component societies regarding evidence-based medicine and dissemination of the products. Most importantly, we solicit proposals for future CPGs and ECSs, and evaluate them for their importance, feasibility, and priority compared with other proposals. Topics are considered for timeliness, value to the Academy membership, and the potential impacts on quality of care and reducing practice variations.
Upcoming CPG and ECS Topics In Queue
The queue for quality products now includes seven CPG topics, seven CPG update topics, and one ECS topic. We recognize the need to address new topics in the queue as well as to perform necessary updates of previously published guidelines to incorporate new knowledge and clinical practices. Our next guideline panels will update the Bell’s Palsy and Tinnitus CPGs, originally published in 2013 and 2014.

Stakeholders Involved in CPG and ECS Development
The GTF relies on the society representatives and other leaders to recruit guideline development panel members with evidence-based medicine interests as well as stakeholder expertise. Our team of methodologists and the AAO-HNSF staff are also crucial to guiding each panel through the creation of a guideline or expert consensus statement from the earliest stages of literature review to the final edits before publication.
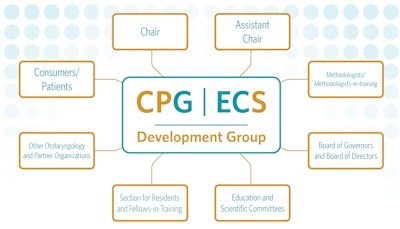
Read the “Role of Panel Members in the Development and Dissemination of Quality Products” to learn more about each of the individual roles and responsibilities.
The Road to Participating in Guideline Development
I have been asked by many colleagues and trainees in otolaryngology about the road to participating in the guideline process. One avenue is by participating in a dedicated program; the AAO-HNSF and GTF have sponsored the Cochrane Scholars and Guidelines International Network (GIN) Scholars programs to provide opportunities for training in structured literature review and guideline development.
In September 2023, Victoria S. Lee, MD, Matthew R. Nauheim, MD, MBA, and John D. Cramer, MD, attended the Cochrane Colloquium in London as Cochrane Scholars. We anticipate selecting scholars to attend the 2024 Global Evidence Summit in Prague, Czech Republic, this coming September. These programs are roads to becoming chairs, assistant chairs, and methodologists for our guideline panels.

CPG and ECS panel members are selected by stakeholder society and committee leaders, and not by the GTF. I encourage interested individuals to make their interests in evidence-based medicine, quality products, and guideline development known to their society and committee leaders. Additionally, we encourage past guideline panel members to consider serving in leadership positions on future panels as chairs, assistant chairs or methodologists.
Making Sure All Voices Are Heard
In my recent discussion with several first-time guideline development group members, a representative from the Society of Otorhinolaryngology and Head-Neck Nurses (SOHN) was impressed with the process and noted, “There was truly an effort to make sure all voices were heard, and all opinions explored.” This panel member not only authored supportive text for one of the action statements, but also participated in a post-publication podcast that is key to dissemination of the CPG recommendations. An otolaryngologist on a different guideline panel noted, “The process was really smooth [and] well organized,” and also offered a suggestion to standardize how we include health equity considerations in the guideline process.
Upcoming Transitions and Looking Ahead
The GTF will undergo a transition this year as I conclude a seven-year term as the GTF Chair. I have had the pleasure of serving with the kind wisdom and support of past GTF Chairs Seth R. Schwartz, MD, MPH, and Richard M. Rosenfeld, MD, MPH, MBA, as well as alongside our invaluable AAO-HNSF Research and Quality staff. I welcome incoming GTF Chair Margo K. Mckenna, MD, and look forward to continued GTF productivity and collaboration under her leadership.
Finally, I urge more Academy members to participate actively in the guideline development process. Academy members can propose topics for CPGs and ECSs, serve on development panels, and review draft documents as peer reviewers or during public comment. Most importantly, please be sure to read each document and consider how the action statements in the CPGs and statements that achieved consensus in the ECSs can benefit your practice and your patients.
Resources
Rosenfeld RM, Nnacheta LC, Corrigan MD. Clinical consensus statement development manual. Otolaryngol Head Neck Surg. 2015; 153(2 suppl): S1- S14.
Rosenfeld RM, Shiffman RN, Robertson P. Clinical practice guideline development manual, third edition: a quality-driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013; 148(1): S1– S55.
AAO-HNS. 2023. “Role of Panel Members in the Development and Dissemination of Quality Products.” Bulletin. American Academy of Otolaryngology-Head and Neck Surgery. https://bulletin.entnet.org/research-quality/article/22865137/role-of-panel-members-in-the-development-and-dissemination-of-quality-products