Difficult Cochlear Implantation
In the earlier days of the field of implantation, cochlear malformations and ossification were considered a contraindication to surgery.
Daniel H. Coelho, MD, and J. Thomas Roland, Jr., MD
While rewarding, implantation of ossified and dysplastic cochlea presents many unique challenges to both the surgeon and programming team. Altered embryology and physiology of these labyrinthine dysplasias may result in forms and functions unfamiliar to those casually involved with cochlear implants. However, with a thorough understanding of the specific issues attendant to this fascinating and varied patient population, the goal of successful implantation and performance has become a reality.
Advanced cochlear implantation includes both ossified and malformed cochleae. Although there are substantial principal areas of overlap between the two with respect to radiographic workup, electrode choice, and surgical technique, they are in fact two separate conditions, each with their own unique problems and solutions.
Timely and accurate diagnosis are paramount in this patient population, as earlier implantation (especially in the case of post-meningitic hearing loss) leads to improved performance. Both computed tomography (CT) and magnetic resonance imaging (MRI) may be necessary in this patient population. Despite controversies in nomenclature, inner ear dysplasias are readily identifiable by thin section high resolution CT scan of the temporal bone. Likewise, T2-weighted MRI can be helpful evaluating cochlear patency and can be particularly useful in determining the presence of septations within a cavity. MRI is especially important when no reliable auditory thresholds are obtainable with behavioral/play audiometry. In these patients, the presence or absence of a cochlear nerve can be ascertained by evaluating high-quality sagittal views of the internal auditory canal (IAC) in the constructive interference in steady state (CISS)/FIESTA sequences.
The ideal electrode in both normal and abnormal cochleae is one that lies in closest proximity to the neuroepithelium. For ossified cochleae, more rigid arrays can be helpful in navigating the tight architecture of the cochlear lumen. In cases when incomplete insertion of full arrays cannot be achieved, compressed or double arrays can be employed (and should be ordered in advance). In malformed cochleae, the critical neuronal sensory cells don’t necessarily lie in the normal perimodiolar location close to the scala tympani. Moreover, the cochlear neuronal count can in some cases be a fraction of the normal count of 20,000 and still provide meaningful auditory input.
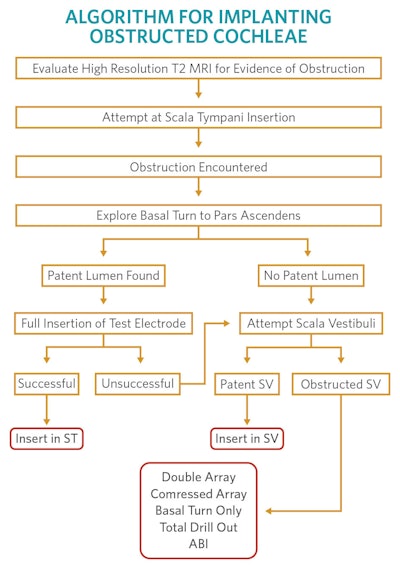
Short or compressed arrays should be utilized in HC patients. The increased risk of complications, including extracochlear placement, intrameatal array insertion, electrode kinking or bending, or insertional trauma resulting in cochlear neuroepithelial damage must all be minimized to achieve optimal placement and performance. In the rare cases of no auditory percept, auditory brainstem implantation may be considered.
Surgical approach should be planned well in advance and is not recommended for the novice surgeon. Of course, approach depends on the particular challenge involved. For ossified cochleae, we recommend the algorithm presented here. For the majority of implantable inner ear malformations, including HC, a standard transmastoid facial-posterior tympanostomy (facial recess) approach to the middle ear can be applied. In many cases, removal of the incus bar and incus may be necessary to provide adequate visualization of optimal cochleostomy target.
The main exception to this is the CC malformation, which is best approached through a simple transmastoid labyrinthotomy. These patients are at risk for several complications, including electrode rollover, facial nerve injury, cerebrospinal fluid leaks, and internal auditory canal (meatal) insertion. We highly recommend the use of intraoperative fluoroscopy to mitigate these risks.
Postoperatively, clear communication between surgeon and implant audiologist is critical. Knowing electrode choice and implanted location are essential to successful programming and usage. The absence of normal tonotopic cochlear architecture can result in unpredictable frequency representation along the electrode. In double-array implantation, the surgeon must know if the apical array is inserted in an anterograde or retrograde fashion.
Just as with surgical considerations, there are a number of programming challenges that present as a result of the abnormal cochlear anatomy and, in particular, the location of neural elements. Successful usage of an implant is related to establishment of a usable program or “MAP.” In patients with malformed cochlea, threshold and comfort levels fluctuate more; therefore, frequent adjustment in programming of these implants is necessary. Likewise, wider stimulation pulse width modes may be needed and hence more power consumed. Stimulation of the facial nerve by the cochlear implant electrodes is common and deactivation of the electrodes in question may be necessary to remove this problem. Though necessary, the deactivation can result in further degradation of the signal available to the neural elements. As a result, MAPping children with anomalous cochleovestibular systems is more difficult and takes longer to achieve. However, within the published literature, there is a consensus that the speech perception results in malformed cochleae obtained over time can in some cases be in the same range as results observed in implant recipients with normally formed cochleae.
Implantation of obstructed and malformed cochleas presents a challenging yet rewarding endeavor for the implant team. Remarkable developments in diagnosis, electrode design, processing strategies, and programming have all contributed to the ability to successfully implant patient populations previously excluded. With increased surgeon comfort in anatomy and technique, more and more children can benefit from this life-changing intervention.
References:
Coelho DH, Roland JT Jr. Implanting obstructed and malformed cochleae. Otolaryngol Clin North Am. 2012 Feb;45(1):91-110. doi: 10.1016/j.otc.2011.08.019. PMID: 22115684
Roland JT Jr, Coelho DH, Pantelides H, Waltzman SB. Partial and double-array implantation of the ossified cochlea. Otol Neurotol. 2008 Dec;29(8):1068-75. doi: 10.1097/MAO.0b013e318188e8ea. PMID: 18833022.
Coelho DH, Waltzman SB, Roland JT Jr. Implanting common cavity malformations using intraoperative fluoroscopy. Otol Neurotol. 2008 Oct;29(7):914-9. doi: 10.1097/MAO.0b013e3181845827. PMID: 18667936