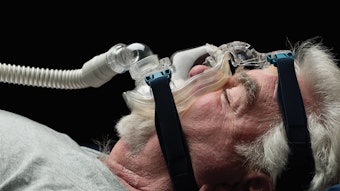
Out of Committee: Airway and Swallowing | Dysphagia in Alzheimer's Disease
Alzheimer’s Disease (AD) is a universally fatal, progressive neurocognitive disease.

Alzheimer’s Disease (AD) is a universally fatal, progressive neurocognitive disease. The prevalence of AD is rising, and 6.2 million persons in the United States and 24 million worldwide are effected.1-3 The incidence of AD is expected to double by 2050.1 AD strikes women more often than men and disproportionally affects Black and Hispanic persons secondary to socioeconomic, environmental, and healthcare disparities.1 AD currently costs the U.S. $355 billion per year in healthcare expenses, of which $76 billion comes directly from the patient and their family.1,4 It is the sixth leading cause of death in the U.S.1 A diagnosis of dysphagia carries a risk of increasing hospital stay length by 40% and may increase costs by $550 million per year.5 Aspiration pneumonia accounts for 6% of AD hospital admissions and is the leading cause of death in AD patients.1,2,4
Dysphagia, even when subclinical, may be present in 50%-75% of early AD patients,3 rising to 84%-93% in moderate-to-severe AD patients.2,6 With use of videofluoroscopic swallow study (VFSS), dysphagia may be diagnosed in up to 95% of AD patients.6 The primary etiology of AD dysphagia is related to the tau neurofibrillary tangles disrupting the cortical areas of the swallowing system.7 The primary cortical areas involved include insula, frontal anterior cingulate cortex, primary and supplementary motor cortex areas, and sensory cortical areas.3,8 Somatic-voluntary nervous system involvement impairs oral preparation, oral phase, oral transit, and oropharyngeal stages of swallowing.9,10 Meanwhile, autonomic nervous system impairment decreases saliva production as well as pharyngeal and supraglottic sensation, increases residue accumulation, and reduces smooth muscle esophageal motility.9-11 Risk of penetration and aspiration increases as AD advances.9,10 Cognitive impairment, sarcopenia, postural instability, and central hypotonia also worsen with AD progression.6
Dysphagia progresses as severity from subclinical early in AD to overt with moderate AD and eventually to apraxia and tactile-oral agnosia.2,3,6-8 With progression of dysphagia from thin liquids to solids (early-to-severe AD), AD patients risk dehydration, malnutrition, aspiration pneumonia, and death.2,4,7,11 The significant morbidity and mortality of dysphagia in AD warrants early and frequent assessment while the insidious neurocognitive decline of AD provides significant diagnostic and therapeutic challenges.
Diagnosing Dysphagia in Alzheimer’s Disease
As early as the onset of AD, patients may start to have difficulty with swallowing. During the course of the illness, the swallowing function becomes altered in multiple phases. Oral transit time becomes delayed as well as the pharyngeal response—these in conjunction with the decreased ability to recognize food visually, oral-tactile agnosia, swallowing and feeding apraxia result in a hindrance of food intake leading to multiple adverse consequences.12 It is imperative to detect the initial signs of dysphagia so that patients can be properly managed.
There are three main diagnostic approaches for the assessment of dysphagia in AD. The first is a non-invasive approach with clinical swallow evaluation. The clinical swallow evaluation is an assessment based on both swallowing problem questionnaires completed by the caregiver of the patient and a motor/sensory examination of all oral structures that complete bolus formation and a swallowing evaluation of liquids and solids of different consistencies. The clinical swallow examination is helpful in assessing for oral praxis, improper laryngeal elevation, lack of gag and pharyngeal reflexes, dysphonia, and the patient’s swallow attempts.2 In addition to the clinical evaluation of swallow, a comprehensive geriatric assessment (CGA) should be considered. The CGA is a multidisciplinary diagnostic process that identifies medical, psychosocial, and functional limitations in the geriatric population in order to develop a plan that maximizes the patient’s health with aging.2
The second approach is to obtain an instrumental assessment, either a VFSS or a fiberoptic endoscopic evaluation of swallowing (FEES). The VFSS helps analyze the bolus preparation, oral transit time, pharyngeal swallow initiation time, hyolaryngeal excursion, laryngeal penetration, and tracheal aspiration.9 Several studies demonstrate that mild, moderate, and severe AD have significant findings on VFSS, typically starting with prolonged oral transit time and escalating to frank aspiration.1,12,13 FEES is less commonly instituted to examine AD patients. This may be secondary to decreased cognitive ability and compliance with the flexible laryngoscopy. In the only study using FEES to assess dysphagia in AD patients, the authors reported that the patients who were not orientated were at a higher risk of aspiration than those patients who were.14
When there is a cognitive or communication issue with an AD patient, VFSS and FEES may be difficult to employ. There is a growing body of literature about the development of non-invasive electrophysiological swallow tests.3,15,16 The dysphagia limit (DL) test combines electrophysiologic monitoring of the submental/suprahyoid areas with consecutively increasing volumes of water given to the patient. A normal DL is more than 20 ml. DL was noted to be significantly reduced in patients with AD—45% of AD patients with mild disease, 85% with moderate disease, and 89% with severe disease were found to have abnormal DL. This demonstrates that dysphagia can develop early in the disease process and become progressively worse as the disease evolves.
Management of Dysphagia in Alzheimer’s Disease
As mentioned, dysphagia has significant consequences due to malnutrition, weight loss, dehydration, and aspiration pneumonia leading to lengthy hospital stays and mortality. The main goal is avoidance of these complications while maintaining the quality of life of the patient. Of utmost importance is to discuss long-term goals with patients when their cognitive status allows them to make informed decisions and they are able to express their wishes about invasive measures, such as feeding tube placement. Unfortunately, as otolaryngologists, we are generally consulted at late stages of the disease, frequently when patients cannot make these decisions and we have to rely on input from caregivers. As we will continue to see increasing numbers of patients with AD in our clinics, two very useful resources include a systematic review by Alagiakrishnan et al., discussing oropharyngeal dysphagia evaluation and management in various types of dementia, and the review paper by Goldberg et al. Reporting that feeding tube placement largely happens when there is an acute hospitalization due to pneumonia or weight loss, these papers review the outcomes of enteral feeding in detail.17,18 Kuo et al., evaluating the incidence of feeding tube insertions in nursing home residents, report an overall one-year mortality rate of 64.1% with a median survival of 56 days post insertion of tube. Furthermore, 20% of these patients require a transfer back to hospital for tube-related complications.19 Similar outcomes are reported by other studies showing 58% of enterally-fed nursing home patients with dementia suffer from aspiration pneumonia as compared to 16% of those fed orally.20 Multiple studies corroborate these findings and point clearly to the detriment of feeding tube insertion. While there is no survival benefit with its insertion, the 30-day and 12-month mortality rates are higher compared to patients who do not receive feeding tubes.21-23 When counseling patients, the above findings are important to note, including the fact that feeding tubes do not prevent aspiration pneumonia.
As otolaryngologists, most of our efforts will focus on diet modification, postural modifications, and swallowing therapy. Possibly one of the most important components of the initial evaluation is the assessment of polypharmacy. Medications that result in xerostomia, sedation, or cognitive decline should be discontinued if possible. Diet modifications are made depending on VFSS results, and during the earlier stages of the disease, swallowing therapy can be employed successfully. Tang et al. have shown that neuromuscular electrical stimulation and electromyographic feedback can be successful in improving dysphagia in AD patients.24
In conclusion, Alzheimer’s Disease is a complicated, progressive neurological disease that is now one of the leading causes of death in the U.S. Due to the increasing prevalence, we need to equip ourselves with evidence-based knowledge to be able to guide patients and families through many difficult medical, ethical, and moral decisions.
References
1. 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021 Mar;17:327-406.
2. Boccardi V, Ruggiero C, Patriti A, et al. Diagnostic assessment and management of dysphagia in patients with Alzheimer's disease. J Alzheimers Dis. 2016;50:947-955.
3. Seçil Y, Arıcı Ş, İncesu TK, et al. Dysphagia in Alzheimer's disease. Neurophysiol Clin. 2016;46:171-178.
4. Tian H, Abouzaid S, Sabbagh MN, et al. Health care utilization and costs among patients with AD with and without dysphagia. Alzheimer Dis Assoc Disord. 2013;27:138-144.
5. Altman KW, Yu GP, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136:784-789.
6. Özsürekci C, Arslan SS, Demir N, et al. Timing of dysphagia screening in Alzheimer's dementia. JPEN J Parenter Enteral Nutr. 2020;44:5165-24.
7. Douglas VC, Josephson SA. Dementia and Systemic Disease. In: Aminoff MJ and Josephson SA, ed. Aminoff’s Neurology and General Medicine, 6th ed. Cambridge, MA: Academic Press of Elsevier; 2021: 1099-1110.
8. Humbert IA, McLaren DG, Kosmatka K, et al. Early deficits in cortical control of swallowing in Alzheimer's disease. J Alzheimers Dis. 2010;19:1185-1197.
9. Affoo RH, Foley N, Rosenbek J, et al. Swallowing dysfunction and autonomic nervous system dysfunction in Alzheimer's disease: a scoping review of the evidence. J Am Geriatr Soc. 2013;61:2203-2213.
10. Mehraban-Far S, Alrassi J, Patel R, et al. Dysphagia in the elderly population: a videofluoroscopic study. Am J Otolaryngol. 2021;42:102854.
11. Nawaz S, Tulunay-Ugur OE. Dysphagia in the older patient. Otolaryngol Clin North Am. 2018;51(4):769-777.
12. Priefer BA, Robbins J. Eating changes in mild stage Alzheimer’s disease. A pilot study. Dysphagia 1997;12:212-221.
13. Horner J, Alberts MJ, Dawson DV, Cook GM. Swallowing in Alzheimer’s disease. Alzheimer Dis Assoc Discord 1994;8:177-189.
14. Leder SB, Suiter DM, Lisitano Warner H. Answering orientation questions and following single step verbal commands: effects on aspiration status. Dysphagia 2009;24:290-295.
15. Ertekin C, Aydogdu I, Yuceyar N. Piecemeal deglutition and dysphagia limit in normal subjects and in patients with swallowing disorders. J Neurol Neurosurg Psychiatry 1996;61:491-496.
16. Gürgör N, Arici Ş, Incesu TK, et al. Am electrophysiological study of the sequential water swallowing. J Electromyogr Kinesiol 2013;23:619-626.
17. Alagiakrishnan K, Bhanji RA, Kurian M. Evaluation and management of oropharyngeal dysphagia in different types of dementia: a systematic review. Arch Gerontol Geriatr 2013;56:1-9.
18. Goldberg LS, Altman KW. The role of gastrostomy tube placement in advanced dementia with dysphagia: a critical review Clin Interv Aging 2014;9:1733-1739.
19. Kuo S, Rhodes RL, Mitchell SL, et al. Natural history of feeding-tube use in nursing home residents with advanced dementia. J Am Med Dir Assoc 2009;10,264–70
20. Peck A, Cohen CE, Mulvihill MN. Long-term enteral feeding of aged demented nursing home patients. J Am Geriat Soc, 1990;38:1195-1198.
21. Sanders DS, Carter MJ, D’Silva J, et al. Survival analysis in percutaneous endoscopic gastrostomy feeding: a worse outcome in patients with dementia. Am J Gastroenterol. 2000;95:1472–1475.
22. Mitchell SL, Kiely DK, Lipsitz L. The risk factors and impact on survival of feeding tube placement in nursing home residents with severe cognitive impairment. Arch Int Med. 1997;157:327–332.
23. Nair S, Hertan H, Pitchumoni CS. Hypoalbuminemia is a poor predictor of survival after percutaneous endoscopic gastrostomy in elderly patients with dementia. Am J Gastroenterol. 2000;95:133–136.
24. Tang Y, Lin X, Lin XJ, et al. Therapeutic efficacy of neuromuscular electrical stimulation and electromyographic biofeedback on Alzheimer's disease patients with dysphagia Medicine (Baltimore). 2017;96:e8008.