From the Education Committees: Skin Prick Testing for Environmental Allergens
Allergic rhinitis is a common problem and is estimated to impact approximately 35% of the general population. With such a high prevalence and multiple manifestations of the disease in the head and neck, it is not a surprise that allergic rhinitis is encountered frequently by otolaryngologists.
Christopher Brook, MD, for the Rhinology and Allergy Education Committee
Allergic rhinitis is a common problem and is estimated to impact approximately 35% of the general population.1 With such a high prevalence and multiple manifestations of the disease in the head and neck, it is not a surprise that allergic rhinitis is encountered frequently by otolaryngologists. One survey found that about 54% of otolaryngology practices provide immunotherapy either in subcutaneous or sublingual form for their patients.2 The diagnosis of allergy is made by taking a focused history with association of allergic triggers and symptoms. In the absence of a historical diagnosis, that value of specific allergy testing is limited given that allergen sensitization often occurs in patients without allergy symptoms.3
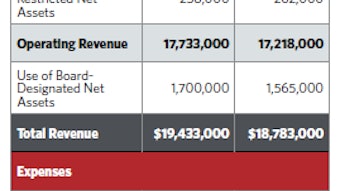
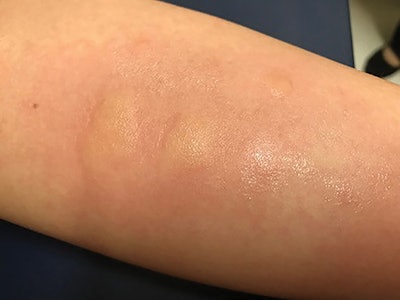 Figure 1. Wheal responses seen after skin prick testing.
Figure 1. Wheal responses seen after skin prick testing.Allergy testing techniques are widely debated, with practitioners diagnosing, treating, and prescribing immunotherapy based on skin prick testing, in vitro testing, and intradermal testing.4 All three techniques provide different advantages and disadvantages and can be used to safely test and treat aero-allergen sensitivities effectively. Skin prick testing uses an application device that pierces the epidermis and applies a small amount of allergen. A whealing response is then read 15–20 minutes later to determine the results (Figure 1). Intradermal testing involves application of antigen directly into the dermis, which increases the sensitivity of testing but can also lead to more discomfort and higher rates of false positive testing. Intradermal testing can be performed in a dilutional technique and can also be performed in a blended technique (known as modified quantitative testing or MQT) with skin prick testing to quantitate the degree of sensitivity and identify a safe starting dose for immunotherapy. Finally, in vitro testing can be used to measure the amount of circulating IgE antibodies to a specific antigen.5
Skin prick testing is the most commonly used technique in most allergy and otolaryngology practices according to surveys,6 likely stemming from ease of use and immediate results gained after testing. There is ample evidence that skin prick testing is accurate for specific allergy testing, faster than intradermal testing, more comfortable than intradermal testing, less likely to cause systemic reactions, and cheaper than in vitro testing.3 Disadvantages are that there is less sensitivity than intradermal dilutional testing and that skin prick testing does not necessarily provide information on the relative degree of sensitivity.3 By basing immunotherapy on skin prick testing alone, assigning an “endpoint” to determine the starting dose of immunotherapy can be challenging due to the nonquantitative nature. Therefore, many practitioners utilize a very conservative starting dose or “endpoint” that is considered safer but leads to longer periods of escalation during immunotherapy treatment (due to a more conservative starting dose).
Practitioners must make their own decisions regarding the tradeoffs between rapid and safe skin prick testing but longer periods of escalation to get to maintenance immunotherapy dosing. This is in contrast to intradermal testing or MQT, which provides more quantitative starting points (and therefore faster escalation) but is more time-consuming to perform. This can also be contrasted with in vitro testing, which is more expensive and requires a delay between testing and obtaining results
Ultimately, the decision of testing technique lies with the individual practitioner, and all forms discussed in this article are acceptable with their own advantages and disadvantages.5
References
- Roxbury CR, Qiu M, Shargorodsky J, Lin SY. Association between allergic rhinitis and poor sleep parameters in U.S. adults. Int Forum Allergy Rhinol. 2018;8(10):1098-1106.
- Leatherman, B, Skoner DP, Hadley JA, et al. The Allergies,Immunotherapy, and Rhinoconjunctivitis (AIRS) survey: provider practices and beliefs about allergen immunotherapy. Int Forum Allergy Rhinol. 2014;4(10):779-788.
- Zuberbier T, Bachert C, Bousquet PJ, et al. GA(2) LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy. 2010;65(12):1525–1530.
- Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108-352.
- Platt MP, Wulu JA. Rational approach to allergy testing. Otolaryngol Clin North Am. 2017;50(6):1103-1110. doi: 10.1016/j.otc.2017.08.007.
- Blaiss MS, Dykewicz MS, Skoner DP, et al. Diagnosis and treatment of nasal and ocular allergies: the Allergies, Immunotherapy, and RhinoconjunctivitiS (AIRS) surveys. Ann Allergy Asthma Immunol. 2014;112(4):322-328.e1. doi: 10.1016/j.anai.2014.02.006.