Out of Committee: Medical Devices and Drugs Update on Oral Appliances for Obstructive Sleep Apnea
Ofer Jacobowitz, MD, PhD ENT and Allergy Associates Assistant Professor, Mount Sinai School of Medicine and Anand K. Devaiah, MD Associate Professor, Departments of Otolaryngology, Neurological Surgery, and Ophthalmology Boston University School of Medicine and Boston Medical Center Obstructive Sleep Apnea (OSA) is a highly prevalent disease, associated with cardiovascular morbidity, mortality, and reduced quality of life. While Positive Airway Pressure (PAP) is efficacious for resolving the airway obstructions that define OSA, many patients are unable to adhere to PAP therapy. Surgical reconstruction can reduce the Apnea Hypopnea Index (AHI), improve quality of life and survival, but many patients defer surgery. Oral appliance therapy has emerged as an efficacious, non-invasive treatment modality for OSA, often preferred to PAP. Oral appliances that advance the mandible have been shown to be effective for OSA treatment. The mechanism of action includes upper-air dilation and stabilization that occurs via tongue advancement and pharyngeal wall lateralization. In recent studies, oral appliances effectiveness was demonstrated for reduction of the AHI and sleepiness, improvement of quality of life and driving performance, and cardiovascular event reduction, even for those with severe OSA (Anandam, Respirology 2013; Doff, Sleep 2013; Holley, Chest 2011; Phillips, AJRCCM 2013). Clinical outcomes were comparable to those achieved with CPAP but AHI reduction was greater with CPAP therapy. New Technologies Two recent technologies may improve outcome for oral appliance therapy. A limitation of appliances for OSA is that the optimal mandibular advancement distance for a patient is not known. Thus, to test treatment efficacy, the patient usually undergoes a polysomnogram or home sleep study once symptoms improve at a given advancement setting. If the result is inadequate, the test may need to be repeated after further adjustment. For some appliances and in certain sleep centers, during the sleep study the technician enters the room and advances the appliance once or twice to determine the better position. Thus, efficacy is determined only after appliance delivery and somewhat imprecisely. A device called MATRx (Zephyr Sleep Technologies) was developed for use at the sleep center, advancing the mandible using an oral tray that stays in the patient’s mouth during sleep. The device is remote-controlled to advance the mandible in fine increments in order to determine the precise protrusion distance for efficacy and whether jaw advancement would be effective at all, prior to production of a costly custom-fitted appliance. The assessment of oral appliance therapy has also been limited by the absence of objective compliance monitoring. Objective monitoring is now available. A tiny data-recording device that attaches to an oral appliance, called DentiTrac (Braebon Medical), allows for assessment of compliance using temperature and position sensors. The manufacturer states that extensive anti-deception algorithms are utilized in data processing so that, for example, placing the device in a bath would be detected. Additional companies are seeking approval at present for other compliance monitoring devices. Compliance monitoring for oral appliances may increase their acceptance as an OSA treatment modality by the transportation industry that requires adherence monitoring for its drivers. Coding Commercial insurance carriers usually cover treatment of OSA with oral appliances. Most policies allow for oral appliances to be used as primary therapy, based on patient preference over CPAP, for patients with mild to moderate OSA, as defined by an AHI between five and 30 events per hour. For patients with severe OSA, as noted by AHI >30/hour, CPAP must be tried first, but oral appliances are usually covered for CPAP intolerant patients. Oral appliances may be billed under two HCPCS codes: E0485 and E0486. E0485 is used for prefabricated, commonly thermoplastic appliances, which are heated and molded to the patient’s dentition in the office. The CMS definition is below: “A prefabricated oral appliance (e0485) is one, which is manufactured in quantity without a specific beneficiary in mind. A prefabricated oral appliance may be trimmed, bent, molded (with or without heat), or otherwise modified for use by a specific beneficiary (i.e., custom-fitted). Any appliance that does not meet the definition of a custom-fabricated oral appliance is considered prefabricated. E0485 is used for all prefabricated oral appliances used for the treatment of OSA including, but not limited to, mandibular advancement devices, tongue positioning appliances, etc.” E0486 is used for a custom-made appliance, laboratory manufactured for a specific patient. This appliance requires taking dental impressions for production and after production is custom-fitted in the office. The CMS definition is below: A custom fabricated oral appliance (E0486) is one that is individually and uniquely made for an individual beneficiary. It involves taking an impression of the beneficiary’s teeth and making a positive model of plaster or equivalent material. Basic materials are cut, bent, and molded using the positive model. It requires more than trimming, bending, or making other modifications to a substantially prefabricated item. A custom fabricated oral appliance may include a prefabricated component (e.g., the joint mechanism). The Otolaryngologist’s Role A qualified otolaryngologist can take impressions, fit, and bill for an oral appliance with most commercial insurance carriers. For CMS, however, when oral appliance therapy became a covered service in 2012, language was introduced specifying that the appliance needs to be provided and billed for by a licensed dentist (DDS or DMD). CMS covers oral appliances for treatment of OSA in patients with mild OSA (AHI five to15) who have symptoms or cardiovascular comorbidities, for patients with moderate OSA (AHI between 15 and 30), and for those with severe OSA (AHI>30) if CPAP is not tolerated or contraindicated. The device needs to be “ordered by the treating physician following review of the report of the sleep test.” There is a 90-day global period for fitting any adjustments. Oral appliances fall under the DME category. When a qualified otolaryngologist provides the oral appliance for an OSA patient with a commercial insurance policy that covers oral appliance therapy, patients will usually be able to afford oral appliance therapy. Most dentists do not take commercial insurance as payment or do not participate with CMS, thus patient access to this therapy is often limited due to prohibitive cost. Otherwise, the otolaryngologist can collaborate with a treating dentist. Patients who use oral appliances need to have a patent nasal airway, as the appliance obstructs their oral airway. The otolaryngologist can assist by optimizing the nasal airway for therapy. FDA Approval There are more than 70 FDA-approved oral appliances. Unfortunately, the device code LRK, “Device, anti-snoring,” includes devices for both treatment of primary snoring and OSA. It is therefore important to check a given oral appliance’s approved indications prior to use. Oral appliances are not FDA-approved for use in severe OSA, and thus their use for severe OSA patients would represent an off-label use. One can check a given device for FDA approval status at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm, or obtain a list of devices where their indications may be checked by searching the web page under product code “LRK” and “LQZ.” Disclosures: Dr. Jacobowitz is an otolaryngologist who is board certified in sleep medicine. He does not have any commercial or competing interests in the devices mentioned above. Dr. Devaiah does not have any relevant disclosures to the topic above. This is another in a series of articles being produced by the Medical Devices and Drugs Committee, written by committee members, consultants, and invited guests for the AAO-HNS membership. Do you have a question or topic we can address, which may fall under the committee’s charge? Do you have a comment about an article? Email our coordinator, Harrison Peery, at hpeery@entnet.org and the chair, Anand Devaiah, MD, at anand.devaiah@bmc.org, with the subject line “MDDC question/article,” so we can identify and answer your query. We may ask your permission to publish your note, in anonymous or edited form, if it becomes the inspiration for a Bulletin article.
Ofer Jacobowitz, MD, PhD
ENT and Allergy Associates
Assistant Professor, Mount Sinai School of Medicine
and
Anand K. Devaiah, MD
Associate Professor, Departments of Otolaryngology, Neurological Surgery, and Ophthalmology
Boston University School of Medicine and Boston Medical Center

Oral appliances that advance the mandible have been shown to be effective for OSA treatment. The mechanism of action includes upper-air dilation and stabilization that occurs via tongue advancement and pharyngeal wall lateralization. In recent studies, oral appliances effectiveness was demonstrated for reduction of the AHI and sleepiness, improvement of quality of life and driving performance, and cardiovascular event reduction, even for those with severe OSA (Anandam, Respirology 2013; Doff, Sleep 2013; Holley, Chest 2011; Phillips, AJRCCM 2013). Clinical outcomes were comparable to those achieved with CPAP but AHI reduction was greater with CPAP therapy.
New Technologies
Two recent technologies may improve outcome for oral appliance therapy. A limitation of appliances for OSA is that the optimal mandibular advancement distance for a patient is not known. Thus, to test treatment efficacy, the patient usually undergoes a polysomnogram or home sleep study once symptoms improve at a given advancement setting. If the result is inadequate, the test may need to be repeated after further adjustment. For some appliances and in certain sleep centers, during the sleep study the technician enters the room and advances the appliance once or twice to determine the better position. Thus, efficacy is determined only after appliance delivery and somewhat imprecisely. A device called MATRx (Zephyr Sleep Technologies) was developed for use at the sleep center, advancing the mandible using an oral tray that stays in the patient’s mouth during sleep. The device is remote-controlled to advance the mandible in fine increments in order to determine the precise protrusion distance for efficacy and whether jaw advancement would be effective at all, prior to production of a costly custom-fitted appliance.
The assessment of oral appliance therapy has also been limited by the absence of objective compliance monitoring. Objective monitoring is now available. A tiny data-recording device that attaches to an oral appliance, called DentiTrac (Braebon Medical), allows for assessment of compliance using temperature and position sensors. The manufacturer states that extensive anti-deception algorithms are utilized in data processing so that, for example, placing the device in a bath would be detected. Additional companies are seeking approval at present for other compliance monitoring devices. Compliance monitoring for oral appliances may increase their acceptance as an OSA treatment modality by the transportation industry that requires adherence monitoring for its drivers.
Coding
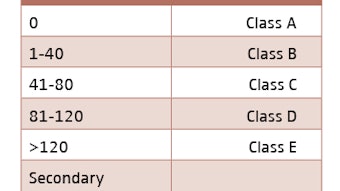
Commercial insurance carriers usually cover treatment of OSA with oral appliances. Most policies allow for oral appliances to be used as primary therapy, based on patient preference over CPAP, for patients with mild to moderate OSA, as defined by an AHI between five and 30 events per hour. For patients with severe OSA, as noted by AHI >30/hour, CPAP must be tried first, but oral appliances are usually covered for CPAP intolerant patients.
Oral appliances may be billed under two HCPCS codes: E0485 and E0486.
E0485 is used for prefabricated, commonly thermoplastic appliances, which are heated and molded to the patient’s dentition in the office. The CMS definition is below:
“A prefabricated oral appliance (e0485) is one, which is manufactured in quantity without a specific beneficiary in mind. A prefabricated oral appliance may be trimmed, bent, molded (with or without heat), or otherwise modified for use by a specific beneficiary (i.e., custom-fitted). Any appliance that does not meet the definition of a custom-fabricated oral appliance is considered prefabricated. E0485 is used for all prefabricated oral appliances used for the treatment of OSA including, but not limited to, mandibular advancement devices, tongue positioning appliances, etc.”
E0486 is used for a custom-made appliance, laboratory manufactured for a specific patient. This appliance requires taking dental impressions for production and after production is custom-fitted in the office. The CMS definition is below:
A custom fabricated oral appliance (E0486) is one that is individually and uniquely made for an individual beneficiary. It involves taking an impression of the beneficiary’s teeth and making a positive model of plaster or equivalent material. Basic materials are cut, bent, and molded using the positive model. It requires more than trimming, bending, or making other modifications to a substantially prefabricated item. A custom fabricated oral appliance may include a prefabricated component (e.g., the joint mechanism).
The Otolaryngologist’s Role
A qualified otolaryngologist can take impressions, fit, and bill for an oral appliance with most commercial insurance carriers. For CMS, however, when oral appliance therapy became a covered service in 2012, language was introduced specifying that the appliance needs to be provided and billed for by a licensed dentist (DDS or DMD). CMS covers oral appliances for treatment of OSA in patients with mild OSA (AHI five to15) who have symptoms or cardiovascular comorbidities, for patients with moderate OSA (AHI between 15 and 30), and for those with severe OSA (AHI>30) if CPAP is not tolerated or contraindicated.
The device needs to be “ordered by the treating physician following review of the report of the sleep test.” There is a 90-day global period for fitting any adjustments. Oral appliances fall under the DME category.
When a qualified otolaryngologist provides the oral appliance for an OSA patient with a commercial insurance policy that covers oral appliance therapy, patients will usually be able to afford oral appliance therapy. Most dentists do not take commercial insurance as payment or do not participate with CMS, thus patient access to this therapy is often limited due to prohibitive cost.
Otherwise, the otolaryngologist can collaborate with a treating dentist. Patients who use oral appliances need to have a patent nasal airway, as the appliance obstructs their oral airway. The otolaryngologist can assist by optimizing the nasal airway for therapy.
FDA Approval
There are more than 70 FDA-approved oral appliances. Unfortunately, the device code LRK, “Device, anti-snoring,” includes devices for both treatment of primary snoring and OSA. It is therefore important to check a given oral appliance’s approved indications prior to use. Oral appliances are not FDA-approved for use in severe OSA, and thus their use for severe OSA patients would represent an off-label use.
One can check a given device for FDA approval status at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMN/pmn.cfm, or obtain a list of devices where their indications may be checked by searching the web page under product code “LRK” and “LQZ.”
Disclosures: Dr. Jacobowitz is an otolaryngologist who is board certified in sleep medicine. He does not have any commercial or competing interests in the devices mentioned above. Dr. Devaiah does not have any relevant disclosures to the topic above.
This is another in a series of articles being produced by the Medical Devices and Drugs Committee, written by committee members, consultants, and invited guests for the AAO-HNS membership. Do you have a question or topic we can address, which may fall under the committee’s charge? Do you have a comment about an article? Email our coordinator, Harrison Peery, at hpeery@entnet.org and the chair, Anand Devaiah, MD, at anand.devaiah@bmc.org, with the subject line “MDDC question/article,” so we can identify and answer your query. We may ask your permission to publish your note, in anonymous or edited form, if it becomes the inspiration for a Bulletin article.