Medical Recalls: What Do We Know and Are We Informed?
Causes of 2024 recalls include safety concerns, software issues, mislabeling, and quality issues.
Quan Lu, and Anita Jeyakumar, MD, MS, on behalf of the Pediatric Otolaryngology Education Committee

- Class I: a situation in which there is a reasonable probability that the use of, or exposure to, a violative product will cause serious adverse health consequences or death.
- Class II: a situation in which use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.
- Class III: a situation in which use of, or exposure to, a violative product is not likely to cause adverse health consequences.
To provide better service in alerting the American people to unsafe, hazardous, or defective products, six federal agencies with vastly different jurisdictions have joined together to create www.recalls.gov – a "one-stop shop" for U.S. Government recalls. The six agencies are the FDA, U.S. Department of Agriculture (USDA), U.S. Environmental Protection Agency (EPA), U.S. Department of Homeland Security, National Highway Traffic Safety Administration (NHTSA), and the U.S. Consumer Product Safety Commission. The FDA has jurisdiction over recalls involving the following: drugs, medical devices, blood and plasma products, vaccines, other biologics, and veterinary products.
A search on the FDA recall site was informative, but did not list some notable recalls, which are described below. (Note that a Google search of each recall did demonstrate an FDA recall.)
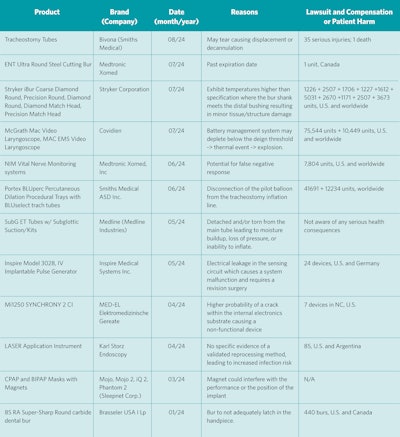
1. Nerve Monitoring System Correction: Medtronic Issued Correction for NIM Vital Nerve Monitoring System due to the Potential for False Negative Response (August 2024).1 This recall involves correcting devices and does not involve removing them from where they are used or sold. The FDA has identified this recall as the most serious type. Medtronic is correcting the NIM Vital Nerve Monitoring System product due to reports of false negative responses. This can occur during procedures, where the device may fail to issue an electromyography (EMG) tone when the NIM probe is placed on a nerve. There have been 10 reported injuries.1
2. An Implantable Hypoglossal Nerve Stimulator Device Removal: Inspire Medical Systems, Inc. Removed Inspire IV Implantable Pulse Generator due to Manufacturing Defect That Can Result in System Malfunctions (July 2024).2 The defect can cause system malfunctions after implantation, leading to electrical leakage in the sensing circuit. As a result, patients may need revision surgery to replace the IPG and restore therapy. The use of affected product may cause serious adverse health consequences, including stimulation below normal therapeutic levels and/or early depletion of the battery (resulting in: loss of therapy), inappropriate or inconsistent stimulation effect, painful stimulation or perceived shocking sensation, and death. There have been no reported injuries. Physicians were advised to “schedule an appointment for the patient to check if their Inspire therapy is working properly by analyzing signals and resistance. Keep an eye out for any changes in the stimulation, lack of therapy effectiveness, or problems with turning the therapy on.”2
3. MED-EL had identified a small number of devices, which may have a higher probability of a crack within the internal electronics substrate which could result in a nonfunctional device (April 2024).3 The recall was originally reported in Canada and was subsequently noted on an FDA site as a Class 2 recall, and seven devices were recalled. It is unclear if patients were affected.4
4. Advanced Bionics (AB) announced a recall of two cochlear implant (CI) models, the "HiRes Ultra" and "HiRes Ultra 3D," because of reports of hearing degradation (February 2020).5 Failures associated with their field corrective action (FCA) reportedly occurred from fluid ingress at the electrode lead causing disruption of electrical signal without compromising the hermetic seal. The defect was discovered during “the device failure analysis” of an implant that was removed on March 26, 2019. Reliability reporting from AB as of March 29, 2021, noted a combined (adult and pediatric) cumulative survival percentage of the Ultra (V1) and Ultra 3D (V1) devices of 92.60% at four years and 98.27% at two years, respectively. An independent study demonstrated the overall failure rate for adults (18.6%) was lower but not significantly different than the pediatric (26.9%) failure rate.5
5. Phillips issued a voluntary recall notification of positive air pressure (PAP) and ventilator devices.6 The recall applies to all Phillips devices manufactured before April 26, 2021, including the original DreamStation line of CPAP machines. (June 2021). The foam within select devices used to diminish noise from the machine may break down into small particles. The particles may then enter the device air pathway and be ingested or inhaled by the PAP user. In addition, the foam may degrade into volatile chemicals (Toluenes) that are potentially toxic. Philips Respironics has stated that 0.03% of patients reported symptoms such as headache, upper airway irritation, cough, chest pressure, and sinus infection, but the manufacturer stated that it has “not received reports of patient impact or serious harm as a result of this issue.”6
How are physicians, other clinicians, and patients notified about recalls, particularly in our current era of large, bureaucratic organizations? Recall notifications can enter different parts of an organization from many directions. For example, the device manufacturer, pharmaceutical company, or FDA may send notifications to a limited or a broad list of contacts. Other communication routes may include manufacturer or vendor letters, mass media coverage, professional society news updates, or word-of-mouth communication from patients and academic peers. These unpredictable streams of information create an opportunity for human error via poorly organized internal communication. One part of the organization may falsely assume that the other is already aware and addressing the situation. Such a lack of coordination can cause reactive delays for the organization and a poorly organized response, potentially causing harm to patients. Poorly coordinated responses may lead to diverse and/or conflicting communication with patients and staff, fueling anxiety and stress.
A publication by the Mayo Clinic regarding the CPAP recall suggests that guided actions were (1) ensuring centralized awareness of device recalls, (2) helping staff to visualize their proactive approaches to the situation, and (3) using empathic communications when informing patients about the recall.7
Please note that this article is not a call for more middle management. Physicians and patients have a unique relationship. Organizations (including manufacturers) need to have a streamlined mechanism to notify both. We all are recipients of blast emails from manufacturers about advances and new releases. The same inherent effort should go into recall notifications. Table 1 summarizes the last year of recalls relevant to otolaryngology. If any of the listed recalls surprise you, and it’s in your field of interest, then there was a breakdown in communication and an opportunity for improvement.
Table 1. Recalls Relevant to Otolaryngology In the Last Year
References
- https://www.fda.gov/medical-devices/medical-device-recalls/nerve-monitoring-system-correction-medtronic-issues-correction-nim-vital-nerve-monitoring-system-due accessed September 30, 2024
- https://www.fda.gov/medical-devices/medical-device-recalls/implantable-hypoglossal-nerve-stimulator-device-removal-inspire-medical-systems-inc-removes-inspire accessed September 30, 2024
- https://recalls-rappels.canada.ca/en/alert-recall/synchrony-2-cochlear-implant accessed September 30, 2024
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfres/res.cfm?id=207499 accessed on September 30, 2024
- Lindquist NR, Cass ND, Patro A, Perkins EL, Gifford RH, Haynes DS, Holder JT. HiRes Ultra Series Recall: Failure Rates and Revision Speech Recognition Outcomes. Otol Neurotol. 2022 Aug 1;43(7):e738-e745. doi: 10.1097/MAO.0000000000003598. PMID: 35878635; PMCID: PMC9335892.
- https://www.enthealth.org/be_ent_smart/phillips-recall-of-pap-devices/ accessed September 30, 2024
- Morgenthaler TI, Linginfelter EA, Gay PC, Anderson SE, Herold D, Brown V, Nienow JM. Rapid response to medical device recalls: an organized patient-centered team effort. J Clin Sleep Med. 2022 Feb 1;18(2):663-667. doi: 10.5664/jcsm.9748. PMID: 34705629; PMCID: PMC8805014.