Balancing access and safety of hearing aids
Diseases of the ear, including hearing loss, have long been a focus for otolaryngologists. Diagnosing, treating, and preventing the various causes of hearing loss has been and remains to be a significant part of the general otolaryngologist’s practice as well as spawning specialty- related practices in neurotology and otology.
James C. Denneny III, MD

The goal of achieving better hearing for as many patients as possible has been a guiding principle for otolaryngologists for centuries. This includes significant commitment to education and research activities designed to define and improve “best practice” paradigms. Inherent to these activities is the promise to advocate for not only the highest quality care, but also access to receive that care.
Events over the last 18 months have signaled a potential marked change in the delivery of hearing healthcare services for individuals 18 years of age and older. Spurred by the persistent low rate of adoption of technologies that would benefit many patients as well as disruptive technology advances, there has been significant interest in removing barriers and improving access for patients to enter the hearing aid market.
Currently in the United States, it is estimated that 20 percent of the individuals who would benefit from hearing aids actually use the technology—a figure that hasn’t changed since 2001. While there is no universal agreement as to the dominant cause for this phenomenon, factors such as cost, social barriers, failure to recognize the problem, denial, and the complexity of navigating the hearing healthcare system itself are certainly contributors. There is a general consensus that both initial and continuing costs are a major factor.
Converging factors, such as the increased availability of medical devices and other treatment options online and over-the-counter (OTC) and the availability of low-cost alternatives that improve hearing, led to the creation of expert panels by the President’s Council of Advisors on Science and Technology (PCAST) and the National Academy of Medicine (NAM). The Academy contributed testimony to each group providing written comments prior to and/or following the release of their respective reports.
The Food and Drug Administration (FDA) also received testimony from interested stakeholders regarding the overall issue, including mandatory medical examinations and OTC sales. I represented the Academy at those hearings and was the only physician giving testimony. The FDA issued an updated guidance in December 2016 (Docket Number FDA-2016-D-3466; “Immediately in Effect Guidance Document: Conditions for Sale for Air Conduction Hearing Aids”), which stated that the agency will no longer enforce the requirement for a medical evaluation/waiver prior to purchasing a hearing aid for adults.
Academy advocacy
The Academy has spent considerable time and resources weighing in on this debate and advocating for our members and patients, as has been our practice and commitment for many years. The earliest testimony regarding hearing aids by the Academy was given in 1973 by Robert J. Ruben, MD, before the Subcommittee on Consumer Interests of the Elderly of the United States Senate. (See related article.) Subsequent input led to the inclusion of package inserts as well as the “red flag warnings for ear disease” issued by the FDA.
I have personally been involved with this issue for more than 20 years, particularly in relation to the red flags warning and the medical examination requirement by the FDA. I was the founding chair of the Coalition for Hearing and Balance (CHB), a collaborative effort between the AAO-HNS, the American Neurotology Society (ANS), and the American Otological Society (AOS). This group’s work on patient education, physician education, and patient advocacy subsequently led to the formation of America’s Hearing Healthcare Team in 2001. This organization included stakeholders in the audiology community and primary care in addition to the CHB.
In reviewing the introductory article in the May 2001 Bulletin, I found the following quote from my original piece: “… in our role as physicians, we have pledged to do our best to give our patients the optimal care, as we understand this is a considerably more burdensome task in the age of rising cost-managed care roadblocks, and widespread economically driven expansion of traditional practice boundaries, but we must not ignore opportunities to fulfill this mission.” This particular statement is equally applicable to today’s times and must be our guiding principle as we move forward.
The question of access
Throughout the recent process, the Academy has sought a balance between access and safety. Certainly, there have been technological advances in hearing aids, but it is disappointing that only 20 percent of those who might benefit have actually adopted the technology. Empirically, one might think that lowering the price and easing the access would reduce the problem. However, when you look at different payment and access systems around the world, it is not clear that that is the case. Japan, which follows an OTC model, has only a 14 percent adoption rate. The United Kingdom, which dispenses hearing aids at no cost to patients, has a 41.1 percent adoption rate. France, which operates under a patient pay model, has a penetration of 30.4 percent, and Germany, which has a mixed model, has 34 percent adoption rate.
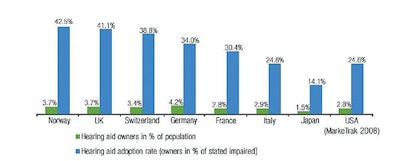 Courtesy of the Hearing Review, March 7, 2013. http://www.hearingreview.com/2013/03/eurotrakjapantrak- 2012-societal-and-personal-benefits-of-hearing-rehabilitation-with-hearing-aids/
Courtesy of the Hearing Review, March 7, 2013. http://www.hearingreview.com/2013/03/eurotrakjapantrak- 2012-societal-and-personal-benefits-of-hearing-rehabilitation-with-hearing-aids/The worldwide experience would indicate adjusting price and availability is not the total solution. In the United States, consumerism and the explosion of information on the internet relating to healthcare problems and solutions is driving some of these proposed changes. Technologies that will allow untrained consumers to assess their own hearing have now advanced to apps that individuals can use to test not only hearing levels but word discrimination. These technologies have the potential to bring patients into the hearing healthcare system who would not have previously participated. A recently released report by the Centers for Disease Control and Prevention (CDC) on noise induced hearing loss (NIHL) highlights the fact that 30 percent of patients with significant NIHL were unaware of this fact (https://www.cdc.gov/vitalsigns/HearingLoss/index.html). The availability of this type of testing will hopefully lead to earlier identification of hearing loss and alert patients of the need to seek medical care.
The question of safety
Current discussion centers around three types of non-surgical devices that have the potential to improve hearing in patients using them. Personal Sound Amplifying Products (PSAPs), entry-level hearing aids, and high-end hearing aids all have shown the ability to improve hearing when placed on individuals whose hearing loss is appropriate for the specific product. PSAPs are not classified as medical devices and are available over-the-counter. The FDA is currently in the process of gathering information and formulating a policy that might allow entry-level hearing aids to be sold over-the-counter. Recommendations by the PCAST and the NAM would support the OTC availability of these types of aids for patients with mild to moderate sensorineural hearing loss. It is unclear when that policy will be released and exactly what it will say. The Academy’s recommendation to the FDA is to allow this to take place but continue to require a medical examination prior to the purchase.
While we support increased access to technologies that can improve hearing for mild to moderate hearing losses, we believe that there are critical patient safety issues that cannot be ignored. Although the majority of patients with hearing loss do not have medically treatable disease, we are all aware of conductive, mixed, and sensorineural hearing loss scenarios that not only can be treated, but are also related to more consequential disease that needs diagnosis and management. We also maintain that for any of these devices to be effective, the type and severity of hearing loss must be accurately determined. Placing a PSAP on a patient with severe to profound hearing loss is inappropriate and ineffective.
Both the FDA and the U.S. Congress are considering actions to increase affordable access to amplification devices and medical devices to improve hearing. We are strongly advocating manufacturing standards to be established and maintained for the PSAPs. There are reports of existing devices with gains of up to 135 dB, and we feel that the patient population needs to be protected from devices that could actually worsen their hearing. In the absence of a requirement for medical examination/waiver, we feel it is essential that package inserts continue to highlight “red flag warnings,” treatable causes of hearing loss, and the advisability of a medical examination.
We are hopeful that recommended changes will actually lead to earlier detection of hearing loss and entry into the hearing healthcare system, where patients will get appropriate treatment on a longitudinal basis. We also are proposing that data be collected both prospectively and retrospectively related to both benefits and the frequency of overlooked treatable causes of hearing loss and the ramifications of that failure. As we have done consistently for decades, we will continue to focus on what is best for our patients.
To receive a copy of an AAO-HNS presentation by James C. Denneny III, MD, “Remediation of Hearing Loss in the United States,” email Bulletin@entnet.org
Better Hearing and Speech Month
For downloadable patient information and relevant material, go to www.entnet.org/BetterHearingSpeechMonth




























