Clinical Practice Guideline: Tinnitus Summary
David E. Tunkel, MD; Carol A. Bauer, MD; Gordon H. Sun, MD, MS; Richard M. Rosenfeld, MD, MPH; Sujana S. Chandrasekhar, MD; Eugene R. Cunningham Jr., MS; Sanford M. Archer, MD; Brian W. Blakely, MD, PhD; John M. Carter, MD; Evelyn C. Granieri, MD, MPH, MSEd; James A. Henry, PhD; Deena Hollingsworth, RN, MSN, FNP; Fawad A. Khan, MD; Scott Mitchell, JD, CPA; Ashkan Monfared, MD; Craig W. Newman, PhD; Folashade S. Omole, MD; C. Douglas Phillips, MD; Shannon K. Robinson, MD; Malcolm B. Taw, MD; Richard S. Tyler, PhD; Richard Waguespack, MD; Elizabeth J. Whamond This month, the American Academy of Otolaryngology—Head and Neck Surgery Foundation (AAO–HNSF) will publish its latest clinical practice guideline, Tinnitus, as a supplement to Otolaryngology–Head and Neck Surgery. Recommendations developed address the evaluation of patients with tinnitus, including selection and timing of diagnostic testing and specialty referral to identify potential underlying treatable pathology, the evaluation and treatment of patients with persistent primary tinnitus, and the most appropriate interventions to improve symptoms and quality of life (QOL) for tinnitus sufferers. The guideline was developed using the a priori protocol outlined in the AAO–HNS Clinical Practice Guideline Development Manual.1 The complete manual is available at http://oto.sagepub.com/content/148/1_suppl/S1.full. To assist in implementing the guideline recommendations, this article summarizes the rationale, purpose, and key action statements. Recommendations in a guideline can only be implemented if they are clear and identifiable. This goal is best achieved by structuring the guideline around a series of key action statements, which are supported by amplifying text and action statement profiles. For ease of reference, only the statements and profiles are included in this brief summary. Please refer to the complete guideline for the important information in the amplifying text that further explains the supporting evidence and details of implementation for each key action statement. For more information about the AAO–HNSF’s other quality knowledge products (clinical practice guidelines and clinical consensus statements), our guideline development methodology, or to submit a topic for future guideline development, visit http://www.entnet.org/guidelines. Introduction Tinnitus is the perception of sound without an external source. More than 50 million people in the United States have reported experiencing tinnitus, resulting in an estimated prevalence of 10 percent to 15 percent in adults.2 About 20 percent of adults who experience tinnitus will require clinical intervention.3 Not a disease in and of itself, tinnitus is actually a symptom that can be associated with multiple causes and aggravating co-factors. Tinnitus is relatively common, but in rare cases it can be a symptom of serious disease such as vascular tumor or vestibular schwannoma (VS). Tinnitus can be persistent, bothersome, and costly. The prevalence of tinnitus was estimated in the National Health Interview Survey conducted in the United States in 1994 by asking whether individuals experienced “ringing, roaring, or buzzing in the ears that lasted for at least three months.”Such tinnitus was present in 1.6 percent of adults age 18-44 years, 4.6 percent of adults age 45-64 years, and 9.0 percent of adults age >60 years.4 In the Beaver Dam offspring study of more than 3,000 adults between the ages of 21 and 84 years studied between 2005 and 2008, 10.6 percent reported tinnitus of at least moderate severity or causing difficulty falling asleep.5 Tinnitus can also have a large economic impact. For example, tinnitus was the most prevalent service-connected disability for U.S. military veterans receiving compensation at the end of fiscal year 2012, resulting in nearly 1 million veterans receiving disability awards.6 Tinnitus can occur on one or both sides of the head and can be perceived as coming from within or outside the head. Tinnitus most often occurs in the setting of concomitant sensorineural hearing loss (SNHL), particularly among patients with bothersome tinnitus and no obvious ear pathology. The quality of tinnitus can also vary, with ringing, buzzing, clicking, pulsations, and other noises described by tinnitus patients. Additionally, the effects of tinnitus on health-related QOL vary widely, with most patients less severely affected but some experiencing anxiety, depression, and extreme life changes. Patients who have tinnitus accompanied by severe anxiety or depression require prompt identification and intervention, as suicide has been reported in tinnitus patients7 who have co-existing psychiatric illness. Most tinnitus is subjective, perceived only by the patient. In contrast, objective tinnitus can be perceived by others, is rare, and is not the focus of this guideline. The focus of this guideline is tinnitus that is bothersome and persistent (lasting six months or longer), often with a negative influence on the patient’s QOL. The guideline development group (GDG) chose six months as the criterion to define persistent tinnitus, since this duration is used most often as an entry threshold in published research studies on tinnitus. Some studies have used tinnitus of three months’duration for eligibility; it is possible that the recommendations of this clinical practice guideline (CPG) may be applicable to patients with tinnitus of shorter duration as well. As noted in Table 1, tinnitus should be classified as either primary or secondary. In this guideline, the following definitions are used: Primary tinnitus is used to describe tinnitus that is idiopathic and may or may not be associated with SNHL. While there is currently no cure for primary tinnitus, a wide range of therapies have been used and studied in an attempt to provide symptomatic relief. These therapies include education and counseling, auditory therapies that include hearing aids and specific forms of sound therapy, cognitive behavioral therapy (CBT), medications, dietary changes and supplements, acupuncture, and transcranial magnetic stimulation. Secondary tinnitus is tinnitus that is associated with a specific underlying cause (other than SNHL) or an identifiable organic condition. It is a symptom of a range of auditory and non-auditory system disorders that include simple cerumen impaction of the external auditory canal, middle ear diseases such as otosclerosis or Eustachian tube dysfunction, cochlear abnormalities such as Meniere’s disease, and auditory nerve pathology such as VS. Non-auditory system disorders that can cause tinnitus include vascular anomalies, myoclonus, and intracranial hypertension. Management of secondary tinnitus is targeted toward identification and treatment of the specific underlying condition, and is not the focus of this guideline. Despite the high prevalence of tinnitus and its potential significant influence on QOL, there are no evidence-based, multidisciplinary CPGs to assist clinicians with management. This guideline attempts to fill this void through actionable recommendations to improve the quality of care that tinnitus patients receive, based on current best research evidence and multidisciplinary consensus. The guideline recommendations will assist clinicians in managing patients with primary tinnitus, emphasizing interventions and therapies deemed beneficial, and avoiding those that are time-consuming, costly, and ineffective. Purpose The purpose of this guideline is to provide evidence-based recommendations for clinicians managing patients with tinnitus. The target audience is any clinician, including non-physicians, involved in managing these patients. Patients with tinnitus will often be evaluated by a variety of healthcare providers including primary care clinicians, specialty physicians, and non-physician providers such as audiologists and mental health professionals. The target patient population is limited to adults (18 years and older) with primary tinnitus that is persistent and bothersome. Tinnitus is often a bothersome, potentially significant complaint of patients with identified causes of hearing loss such as Meniere’s disease, sudden SNHL, otosclerosis, and VS. Patients with these identifiable and other causative diagnoses of secondary tinnitus are excluded from this guideline, as they are often excluded from nearly all randomized controlled trials (RCTs) of tinnitus management, making it impossible to generalize trial results. However, the GDG placed emphasis on the need for thorough clinical evaluation to identify these potentially treatable and sometimes serious disorders. Clinicians should decide whether to apply these recommendations to patients with these conditions on an individualized basis. The guideline also excludes patients with pulsatile tinnitus, or tinnitus related to complex auditory hallucinations or hallucinations related to psychosis or epilepsy. This is the first evidence-based clinical guideline developed for the evaluation and treatment of chronic tinnitus. This guideline provides clinicians with a logical framework to improve patient care and mitigate the personal and social impact of persistent, bothersome tinnitus. It will discuss the evaluation of patients with tinnitus, including selection and timing of diagnostic testing and specialty referral to identify potential underlying treatable pathology. It will then focus on the evaluation and treatment of patients with persistent primary tinnitus, with recommendations to evaluate and measure its influence, as well as for determining the most appropriate interventions to improve symptoms and QOL for tinnitus sufferers. In formulating this guideline, a broad range of topics were identified as quality improvement (QI) opportunities by the GDG. These topics fall into the three broad domains of: assessment, intervention/management, and education. The group further prioritized these topics to determine the focus of the guideline. Key Action Statements STATEMENT 1. HISTORY AND PHYSICAL EXAM: Clinicians should perform a targeted history and physical examination at the initial evaluation of a patient with presumed primary tinnitus to identify conditions that if promptly identified and managed may relieve tinnitus. Recommendation based on observational studies, with a preponderance of benefit over harm. Action Statement Profile Quality improvement opportunity: To promote a consistent and systematic approach to the initial evaluation of the patient with tinnitus Aggregate evidence quality: Grade C, based on observational studies Level of confidence in evidence: Moderate, as few if any studies specifically investigate the diagnostic yield or impact of history and examination on tinnitus patients Benefits: Identify organic, and potentially treatable, underlying causes (e.g., secondary tinnitus); minimize cost and administrative burden through a targeted approach to history and physical examination; streamline care/increase efficiency; improve patient satisfaction; identify patients with primary tinnitus who may benefit from further management (as outlined in this guideline) Risks, harms, costs: None Benefit-harm assessment: Preponderance of benefit Value judgments: Perception by the GDG that tinnitus sufferers may not receive thorough evaluations from clinicians; further perception that many clinicians are unaware of the optimal targeted history and physical examination to evaluate a patient with tinnitus Intentional vagueness: The definition of a “targeted”history and physical examination is elaborated upon in the supporting text Role of Patient Preferences: None Exclusions: None Policy level: Recommendation Differences of opinion: None. STATEMENT 2a. PROMPT AUDIOLOGIC EXAMINATION: Clinicians should obtain a comprehensive audiologic examination in patients with tinnitus that is unilateral, associated with hearing difficulties, or persistent (≥6months). Recommendation based on observational studies, with a preponderance of benefit over risk. Action Statement Profile Quality improvement opportunity: To address potential underutilization of audiologic testing in patients with tinnitus who are likely to have underlying hearing loss and to avoid delay in such diagnosis Aggregate evidence quality:Grade C, based on observational studies Level of confidence in the evidence: Moderate, as literature about the impact of prompt audiologic assessment on tinnitus management is scant Benefits: Prioritize the need for otolaryngologic evaluation (if not already completed) using audiologic criteria; identify hearing loss, which is frequently associated with tinnitus; characterize the nature of hearing loss (conductive, sensorineural, or mixed; unilateral or bilateral); detect hearing loss that may be unsuspected; initiate workup for serious disease that causes unilateral tinnitus and hearing loss (i.e., VS) Risks, harms, costs: Direct cost of examination; access to testing; time Benefit-harm assessment: Preponderance of benefit Value judgments: None Intentional vagueness: The term “prompt”is used to emphasize the importance of ordering a timely test and ensuring it is done, preferably within four weeks of assessment Role of Patient Preferences: Small; patients may participate in decisions regarding timing of audiogram Exclusions: None Policy level: Recommendation Differences of opinion: None. STATEMENT 2b. ROUTINE AUDIOLOGIC EXAMINATION: Clinicians may obtain an initial comprehensive audiologic examination in patients who present with tinnitus (regardless of laterality, duration, or perceived hearing status). Option based on observational studies, with a balance of benefit and harm. Action Statement Profile Quality improvement opportunities: To promote awareness of hearing loss associated with tinnitus, even in patients who do not have unilateral tinnitus or hearing difficulties, and to emphasize that clinicians do not have to wait six months before obtaining an audiogram if deemed appropriate Aggregate evidence quality: Grade C, based on observational studies and prevalence of HL in RCTs of tinnitus therapy Level of confidence in the evidence: High Benefits:Detect a hearing loss not perceived by the patient; SNHL, which is a treatable condition commonly associated with tinnitus; identify patients who may be candidates for sound therapy; identify opportunities for patient counseling/education Risks, harms, costs: Direct costs of audiologic testing; detection of minor audiologic abnormalities leading to potentially unnecessary further testing or referral; inconsistent access to testing Benefit-harm assessment:Equilibrium Value judgments:None Intentional vagueness: None Role of patient preferences: Large role for shared-decision making to proceed with audiologic examination Exclusions: None Policy level: Option Differences of opinion: None. STATEMENT 3. IMAGING STUDIES:Clinicians should not obtain imaging studies of the head and neck in patients with tinnitus, specifically to evaluate the tinnitus, unless they have one or more of the following: tinnitus that localizes to one ear, pulsatile tinnitus, focal neurological abnormalities, or asymmetric hearing loss. Strong recommendation against based on observational studies, with a preponderance of benefit over harm. Action Statement Profile Quality improvement opportunity: Avoid overuse of imaging in patients with a low likelihood of any significant benefit from the imaging Aggregate evidence quality: Grade C, based on observational studies Level of confidence in the evidence: High Benefits: Avoid testing with low yield; avoid harms of unnecessary tests (radiation, contrast, cost); avoid test anxiety; avoid detecting subclinical, incidental findings Risks, harms, costs: Slight chance of missed diagnosis; relatively high costs and limited access to certain types of imaging studies Benefit-harm assessment: Preponderance of benefit Value judgments: GDG made this a strong recommendation against, instead of a recommendation against, based on consensus regarding the importance of avoiding low-yield, expensive tests with potential adverse events in patients with tinnitus Intentional vagueness: Specific imaging studies are specified in the supporting text including: CT, CTA, MRI, MRA Role of patient preferences: None Exclusions: None Policy level: Strong recommendation against Differences of opinion: None. STATEMENT 4. BOTHERSOME TINNITUS: Clinicians must distinguish patients with bothersome tinnitus from patients with non-bothersome tinnitus. Strong recommendation based on inclusion criteria for RCTs on tinnitus treatment, with a preponderance of benefit over harm. Action Statement Profile Quality improvement opportunity: To identify those patients in need of clinical management, and limit unnecessary testing and treatment for others Aggregate evidence quality: Grade B, based on inclusion criteria for RCTs on tinnitus treatment Level of confidence in evidence: High Benefits: Identify patients for further counseling and/or intervention/management; determine impact of tinnitus on health-related QOL; identify patients with bothersome tinnitus who may benefit from additional assessment for anxiety and depression; encourage an explicit and systematic assessment of patients to avoid underestimating or trivializing the impact of tinnitus; avoid unnecessary interventions/management of patients with non-bothersome tinnitus Risks, Harms, Costs: Time involved in assessment Benefit-Harm Assessment: Preponderance of benefit Value Judgments: None Intentional Vagueness: Method of distinguishing bothersome vs. non-bothersome is not specifically stated. One or more of the validated questionnaires described in the supporting text may be helpful Role of Patient Preferences: None Exclusions: None Policy Level: Strong recommendation Differences of opinion: None. STATEMENT 5. PERSISTENT TINNITUS: Clinicians should distinguish patients with bothersome tinnitus of recent onset from those with persistent symptoms (≥6months) to prioritize intervention and facilitate discussions about natural history and follow-up care. Recommendation based on inclusion criteria in RCTs, with a preponderance of benefit over harm. Action Statement Profile Quality improvement opportunity: To identify patients with a duration of tinnitus similar to that studied in RCTs of tinnitus treatment; to identify those who may need and benefit from intervention; and to avoid inappropriate interventions for patients with shorter duration tinnitus Aggregate evidence quality: Grade B, based on inclusion criteria in RCTs Level of confidence in the evidence: Moderate, based on varying tinnitus duration in RCTs, with some including patients with tinnitus of less than three months’duration Benefits: Identify patients who have duration of tinnitus similar to the patients included in RCTs, and identify those patients who are most likely to benefit from intervention Risks, Harms, Costs: Defer treatment that may benefit some tinnitus patients who do not have persistent symptoms Benefit-Harm Assessment: Preponderance of benefit Value Judgments: Despite some variation in inclusion criteria for duration of tinnitus used in clinical trials, the GDG felt that six months was a reasonable time to conclude that the tinnitus would likely persist Intentional Vagueness: None Role of Patient Preferences: None Exclusions: None Policy Level: Recommendation Differences of opinion: None. STATEMENT 6. EDUCATION AND COUNSELING: Clinicians should educate patients with persistent, bothersome tinnitus about management strategies.Recommendation based on studies of the value of education and counseling, with a preponderance of benefit over harm. Action Statement Profile Quality improvement opportunity: To address potential underutilization of education and counseling by clinicians who manage patients with persistent, bothersome tinnitus. To bring awareness of available management strategies to the patient Aggregate evidence quality: Grade B, based on studies of the value of education and counseling in general, and Grade C based on such studies in tinnitus in particular Level of confidence in the evidence: High Benefits: Improved QOL; increased ability to cope with tinnitus; improved outcomes and patient satisfaction; less healthcare utilization Risks, harms, costs: Direct cost and time Benefit-harm assessment: Preponderance of benefit Value judgments: None Intentional vagueness: None Role of patient preferences: None Exclusions: None Policy level: Recommendation Differences of opinion: None. STATEMENT 7. HEARING AID EVALUATION: Clinicians should recommend a hearing aid evaluation for patients with hearing loss and persistent, bothersome tinnitus.Recommendation based on observational studies with a preponderance of benefit over harm. Action Statement Profile Quality improvement opportunities: To promote awareness of the beneficial effect of hearing aids on tinnitus and encourage utilization of this first-line audiologic intervention for patients with tinnitus, even those who might otherwise be marginal hearing aid candidates Aggregate evidence quality: Grade C, based on observational studies Level of confidence in the evidence: High Benefits: Raise awareness of potential beneficial effects of hearing aids on tinnitus; ensure that patient receives proper guidance regarding benefits and costs of hearing aids; provide patients who have hearing loss with access to information and interventions that may alleviate hearing loss and improve function/QOL Risks, harms, costs: direct cost related to dispensing of a hearing aid Benefit-harm assessment: Preponderance of benefit Value judgments: Perceived lack of awareness regarding the ability of hearing aids to improve QOL for patients with tinnitus Intentional vagueness: The level of hearing loss is not specified because hearing loss-associated tinnitus may benefit from hearing aids even if the hearing loss is only of a mild degree, or even if there is a more severe unilateral SNHL associated with the tinnitus Role of patient preferences: Patient may accept or decline the recommendation to pursue a hearing aid evaluation Exclusions: None Policy level: Recommendation Differences of opinion: None. STATEMENT 8. SOUND THERAPY: Clinicians may recommend sound therapy to patients with persistent, bothersome tinnitus. Option based on RCTs with methodological concerns, with a balance between benefit and harm. Action Statement Profile Quality improvement opportunity: To promote awareness and utilization of sound therapy as a reasonable management option in patients with persistent, bothersome tinnitus Aggregate evidence quality: Grade B, based on RCTs with methodological concerns Level of confidence in the evidence: Medium, as strength of evidence is low. Benefits: Access to technology/devices that may relieve tinnitus; improve QOL, sleep, and concentration Risks, harms, costs: Consequences of recommending an intervention of uncertain efficacy; promoting false hope; costs associated with sound therapy Benefit-harm assessment: Equilibrium Value judgments: None Intentional vagueness: None Role of patient preferences: Significant role in deciding whether to pursue sound therapy and to choose among the available options Exclusions: None Policy level: Option Difference of opinion: One GDG member expressed a difference of opinion about mechanisms of sound therapy, in particular with the concepts of partial and total masking. STATEMENT 9. COGNITIVE BEHAVIOR THERAPY (CBT): Clinicians should recommend CBT to patients with persistent, bothersome tinnitus. Recommendation based on RCTs, with a preponderance of benefit over harm. Action Statement Profile Quality improvement opportunity: To promote awareness and utilization of CBT as an effective management option in patients with persistent, bothersome tinnitus Aggregate evidence quality: Grade A, based on multiple systematic reviews of RCTs Level of confidence in the evidence: Moderate, based on concerns about methodology and sample size of trials Benefits: Treatment of depression and anxiety; improved QOL, tinnitus coping skills, and adherence to other tinnitus treatments Risks, harms, costs: Direct cost; time involved (multiple sessions, 1-2 hrs. each); availability to services may be limited Benefit-harm assessment: Preponderance of benefit Value judgments: None Intentional vagueness: None Role of patient preferences: None Exclusions: None Policy level: Recommendation Differences in opinion: None. STATEMENT 10. MEDICAL THERAPY: Clinicians should not routinely recommend antidepressants, anticonvulsants, anxiolytics, or intratympanic medications for a primary indication of treating persistent, bothersome tinnitus.Recommendation against based on systematic reviews and RCTs with methodological concerns, with a preponderance of benefit over harm. Action Statement Profile Quality improvement opportunity: To decrease the use of medications that may have no benefit and have significant potential side effects, in the management of patients with tinnitus Aggregate evidence quality: Grade B, based on RCTs with methodological concerns and systematic reviews demonstrating a low strength of evidence Level of confidence in the evidence: Medium regarding the lack of efficacy of medical therapy as a primary treatment for persistent bothersome tinnitus, as several studies with methodological flaws, bias, and lack of power did show some benefit in certain tinnitus outcome measures Benefits: Avoid unproven therapy, side effects/adverse events (including tinnitus), and false hope; reduce expense. Avoid use of medications that are not approved for use in geriatric population Risks, harms, costs: Denying some patients benefit Benefit-harm assessment: Preponderance of benefit Value judgments: Although these therapies appear to be beneficial in some studies, the evidence from systematic reviews and RCTs is insufficient to justify routine use in managing tinnitus patients, especially given the known harms, cost of therapy, and potential for some medications (e.g., antidepressants) to worsen tinnitus Intentional vagueness: The term “routine”is used to acknowledge there may be individual circumstances for which clinicians and patients may wish to pursue therapy Role of patient preferences: Limited; a trial of medication may be administered based on individual circumstances Exclusions: Patients with depression, anxiety, or seizure disorders that constitute an indication for pharmacologic therapy independent of tinnitus Policy level: Recommendation against Differences in opinion: None. STATEMENT 11. DIETARY SUPPLEMENTS: Clinicians should not recommend Ginkgo biloba, melatonin, zinc, or other dietary supplements for treating patients with persistent, bothersome tinnitus.Recommendation against based on RCTs and Systematic Reviews with methodological concerns, with a preponderance of benefit over harm. Action Statement Profile Quality improvement opportunity: To avoid use of commonly-available supplements that have no proven efficacy and pose potential harm, in the management of patients with tinnitus Aggregate evidence quality: Grade C, RCTs and systematic reviews with extreme heterogeneity; most of the RCTs raise significant concerns regarding methodology and subject selection Level of confidence in the evidence: High confidence regarding potential harm and adverse effects related to these agents, particularly in the elderly population; low confidence in benefits due to methodological concerns and study quality and ability to generalize results to patients with persistent, primary tinnitus Benefits: Avoid unproven therapy, side effects/adverse events (including tinnitus), and false hope; reduce expense Risks, harms, costs: None Benefit-harm assessment: Preponderance of benefit Value judgments: Concern regarding the actual content and dosage of proposed active agents in these preparations, as they are currently packaged OTC. Many of these supplements, not under the regulations of the USFDA, have varying amounts of the “active”agent. The GDG was concerned over the widespread availability for easy purchase of these agents without considering potential drug interactions and adverse events Intentional vagueness: The term “dietary supplements”is used to generalize nutritional and herbal supplements promoted as remedies for tinnitus Role of patient preferences: Limited role Exclusions: None Policy level: Recommendation against Differences in opinion: The majority of the GDG felt there was a clear predominance of harm over benefit; a minority felt there was an equilibrium. None of the group perceived a preponderance of benefit over harm. STATEMENT 12. ACUPUNCTURE: No recommendation can be made regarding the effect of acupuncture in patients with persistent bothersome tinnitus. No recommendation based on poor quality trials, no benefit, and minimal harm. Action Statement Profile Quality improvement opportunity: Limited, to educate patients and providers about the controversies regarding the use of acupuncture for tinnitus Aggregate evidence quality: Grade C, based on inconclusive RCTs and the presence of costs and potential harm with no established benefit with the use of acupuncture for tinnitus Level of confidence in the evidence: Low regarding benefit because of heterogeneity and methodological flaws in the RCTs; high regarding harm or cost, with the understanding that serious harm from acupuncture is rare Benefits: No direct benefits of no recommendation Risks, harms, costs: Cost of acupuncture therapy, time required for therapy, and potential delay in instituting sound therapy or hearing aids Benefit-harm assessment: unknown Value judgments: The poor quality of the data, the limited potential for harm from acupuncture kept the GDG from making a recommendation about acupuncture Intentional vagueness: None Role of patient preferences: Significant role for shared decision making; patients may wish to try acupuncture based on circumstances Exclusions: None Policy level: No recommendation Differences in opinion: Minor: The GDG was divided between making no recommendation and making a recommendation against the use of acupuncture. STATEMENT 13. TRANSCRANIAL MAGNETIC STIMULATION (TMS): Clinicians should not recommend TMS for the treatment of patients with persistent, bothersome tinnitus. Recommendation against based on inconclusive RCTs. Action Statement Profile Quality improvement opportunity: To avoid use of a therapy that has inconclusive efficacy and poses potential financial and physical harm, in the management of patients with tinnitus Aggregate evidence quality: Grade B, based on inconclusive RCTs and systematic reviews that show low strength of evidence Level of confidence in the evidence: High regarding the absence of a long-term (>6 months) benefit of TMS; moderate regarding the absence of a short-term benefit, since a minority of trials demonstrated transient beneficial outcomes, and strength of this evidence is low Benefits: Avoid unproven therapy, side effects/adverse events, and false hope; reduce expense Risks, harms, costs: Denying some patients benefit Benefit-harm assessment: Preponderance of benefit Value judgments: None Intentional vagueness: None Role of patient preferences: Limited Exclusions: Patients with depression or other neurological conditions for which TMS is indicated Policy level: Recommendation against Differences in opinion: None. Disclaimer The clinical practice guideline is provided for information and educational purposes only. It is not intended as a sole source of guidance in managing patients with tinnitus. Rather, it is designed to assist clinicians by providing an evidence-based framework for decision-making strategies. The guideline is not intended to replace clinical judgment or establish a protocol for all individuals with this condition and may not provide the only appropriate approach to diagnosing and managing this program of care. As medical knowledge expands and technology advances, clinical indicators and guidelines are promoted as conditional and provisional proposals of what is recommended under specific conditions but are not absolute. Guidelines are not mandates; these do not and should not purport to be a legal standard of care. The responsible physician, in light of all circumstances presented by the individual patient, must determine the appropriate treatment. Adherence to these guidelines will not ensure successful patient outcomes in every situation. The AAO-HNSF emphasizes that these clinical guidelines should not be deemed to include all proper treatment decisions or methods of care, or to exclude other treatment decisions or methods of care reasonably directed to obtaining the same results. References Rosenfeld RM, Shiffman RN. Clinical Practice Guideline Development Manual: a quality-driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2009; 140(Suppl1). SI-43. Hoffman HJ, Reed GW. Epidemiology of tinnitus. In: Snow JB. Ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker; 2004:16-41. Henry JA, Zaugg TL, Myers PJ, Schechter MA. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends Amplif. 2008;12(3):170-187. Vital and Health Statistics: Current Estimates from the National Health Interview Survey, 1994. Series 10: Data from the National Health Survey No. 193; US Department of Health and Human Services Public Health Service, CDC, National Center for Health Statistics, DHHS Publication No. (PHS) 96-1521. Nondahl DM, Cruickshanks KJ, Huang GH, et al. Tinnitus and its risk factors in the Beaver Dam Offspring Study. Int J Audiol. 2011; 50(5):313-320. US Department of Veterans Affairs. Annual Benefits Report: Fiscal Year 2012. In: Affairs DoV, ed. Washington, DC. 2013. Lewis JE, Stephens SDG, McKenna L. Tinnitus and suicide. Clin Otolaryngol Allied Sci. 1994;19(1):50-54.
David E. Tunkel, MD; Carol A. Bauer, MD; Gordon H. Sun, MD, MS; Richard M. Rosenfeld, MD, MPH; Sujana S. Chandrasekhar, MD; Eugene R. Cunningham Jr., MS; Sanford M. Archer, MD; Brian W. Blakely, MD, PhD; John M. Carter, MD; Evelyn C. Granieri, MD, MPH, MSEd; James A. Henry, PhD; Deena Hollingsworth, RN, MSN, FNP; Fawad A. Khan, MD; Scott Mitchell, JD, CPA; Ashkan Monfared, MD; Craig W. Newman, PhD; Folashade S. Omole, MD; C. Douglas Phillips, MD; Shannon K. Robinson, MD; Malcolm B. Taw, MD; Richard S. Tyler, PhD; Richard Waguespack, MD; Elizabeth J. Whamond

To assist in implementing the guideline recommendations, this article summarizes the rationale, purpose, and key action statements. Recommendations in a guideline can only be implemented if they are clear and identifiable. This goal is best achieved by structuring the guideline around a series of key action statements, which are supported by amplifying text and action statement profiles. For ease of reference, only the statements and profiles are included in this brief summary. Please refer to the complete guideline for the important information in the amplifying text that further explains the supporting evidence and details of implementation for each key action statement.
For more information about the AAO–HNSF’s other quality knowledge products (clinical practice guidelines and clinical consensus statements), our guideline development methodology, or to submit a topic for future guideline development, visit http://www.entnet.org/guidelines.
Introduction
Tinnitus is the perception of sound without an external source. More than 50 million people in the United States have reported experiencing tinnitus, resulting in an estimated prevalence of 10 percent to 15 percent in adults.2 About 20 percent of adults who experience tinnitus will require clinical intervention.3 Not a disease in and of itself, tinnitus is actually a symptom that can be associated with multiple causes and aggravating co-factors. Tinnitus is relatively common, but in rare cases it can be a symptom of serious disease such as vascular tumor or vestibular schwannoma (VS).
Tinnitus can be persistent, bothersome, and costly. The prevalence of tinnitus was estimated in the National Health Interview Survey conducted in the United States in 1994 by asking whether individuals experienced “ringing, roaring, or buzzing in the ears that lasted for at least three months.”Such tinnitus was present in 1.6 percent of adults age 18-44 years, 4.6 percent of adults age 45-64 years, and 9.0 percent of adults age >60 years.4 In the Beaver Dam offspring study of more than 3,000 adults between the ages of 21 and 84 years studied between 2005 and 2008, 10.6 percent reported tinnitus of at least moderate severity or causing difficulty falling asleep.5 Tinnitus can also have a large economic impact. For example, tinnitus was the most prevalent service-connected disability for U.S. military veterans receiving compensation at the end of fiscal year 2012, resulting in nearly 1 million veterans receiving disability awards.6
Tinnitus can occur on one or both sides of the head and can be perceived as coming from within or outside the head. Tinnitus most often occurs in the setting of concomitant sensorineural hearing loss (SNHL), particularly among patients with bothersome tinnitus and no obvious ear pathology. The quality of tinnitus can also vary, with ringing, buzzing, clicking, pulsations, and other noises described by tinnitus patients. Additionally, the effects of tinnitus on health-related QOL vary widely, with most patients less severely affected but some experiencing anxiety, depression, and extreme life changes. Patients who have tinnitus accompanied by severe anxiety or depression require prompt identification and intervention, as suicide has been reported in tinnitus patients7 who have co-existing psychiatric illness. Most tinnitus is subjective, perceived only by the patient. In contrast, objective tinnitus can be perceived by others, is rare, and is not the focus of this guideline.
The focus of this guideline is tinnitus that is bothersome and persistent (lasting six months or longer), often with a negative influence on the patient’s QOL. The guideline development group (GDG) chose six months as the criterion to define persistent tinnitus, since this duration is used most often as an entry threshold in published research studies on tinnitus. Some studies have used tinnitus of three months’duration for eligibility; it is possible that the recommendations of this clinical practice guideline (CPG) may be applicable to patients with tinnitus of shorter duration as well.
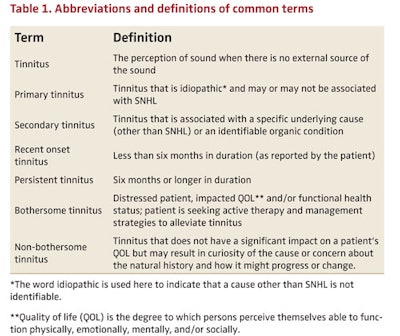
As noted in Table 1, tinnitus should be classified as either primary or secondary. In this guideline, the following definitions are used:
- Primary tinnitus is used to describe tinnitus that is idiopathic and may or may not be associated with SNHL. While there is currently no cure for primary tinnitus, a wide range of therapies have been used and studied in an attempt to provide symptomatic relief. These therapies include education and counseling, auditory therapies that include hearing aids and specific forms of sound therapy, cognitive behavioral therapy (CBT), medications, dietary changes and supplements, acupuncture, and transcranial magnetic stimulation.
- Secondary tinnitus is tinnitus that is associated with a specific underlying cause (other than SNHL) or an identifiable organic condition. It is a symptom of a range of auditory and non-auditory system disorders that include simple cerumen impaction of the external auditory canal, middle ear diseases such as otosclerosis or Eustachian tube dysfunction, cochlear abnormalities such as Meniere’s disease, and auditory nerve pathology such as VS. Non-auditory system disorders that can cause tinnitus include vascular anomalies, myoclonus, and intracranial hypertension. Management of secondary tinnitus is targeted toward identification and treatment of the specific underlying condition, and is not the focus of this guideline.
Despite the high prevalence of tinnitus and its potential significant influence on QOL, there are no evidence-based, multidisciplinary CPGs to assist clinicians with management. This guideline attempts to fill this void through actionable recommendations to improve the quality of care that tinnitus patients receive, based on current best research evidence and multidisciplinary consensus. The guideline recommendations will assist clinicians in managing patients with primary tinnitus, emphasizing interventions and therapies deemed beneficial, and avoiding those that are time-consuming, costly, and ineffective.
Purpose
The purpose of this guideline is to provide evidence-based recommendations for clinicians managing patients with tinnitus. The target audience is any clinician, including non-physicians, involved in managing these patients. Patients with tinnitus will often be evaluated by a variety of healthcare providers including primary care clinicians, specialty physicians, and non-physician providers such as audiologists and mental health professionals. The target patient population is limited to adults (18 years and older) with primary tinnitus that is persistent and bothersome.
Tinnitus is often a bothersome, potentially significant complaint of patients with identified causes of hearing loss such as Meniere’s disease, sudden SNHL, otosclerosis, and VS. Patients with these identifiable and other causative diagnoses of secondary tinnitus are excluded from this guideline, as they are often excluded from nearly all randomized controlled trials (RCTs) of tinnitus management, making it impossible to generalize trial results. However, the GDG placed emphasis on the need for thorough clinical evaluation to identify these potentially treatable and sometimes serious disorders. Clinicians should decide whether to apply these recommendations to patients with these conditions on an individualized basis. The guideline also excludes patients with pulsatile tinnitus, or tinnitus related to complex auditory hallucinations or hallucinations related to psychosis or epilepsy.
This is the first evidence-based clinical guideline developed for the evaluation and treatment of chronic tinnitus. This guideline provides clinicians with a logical framework to improve patient care and mitigate the personal and social impact of persistent, bothersome tinnitus. It will discuss the evaluation of patients with tinnitus, including selection and timing of diagnostic testing and specialty referral to identify potential underlying treatable pathology. It will then focus on the evaluation and treatment of patients with persistent primary tinnitus, with recommendations to evaluate and measure its influence, as well as for determining the most appropriate interventions to improve symptoms and QOL for tinnitus sufferers.
In formulating this guideline, a broad range of topics were identified as quality improvement (QI) opportunities by the GDG. These topics fall into the three broad domains of: assessment, intervention/management, and education. The group further prioritized these topics to determine the focus of the guideline.
Key Action Statements
STATEMENT 1. HISTORY AND PHYSICAL EXAM: Clinicians should perform a targeted history and physical examination at the initial evaluation of a patient with presumed primary tinnitus to identify conditions that if promptly identified and managed may relieve tinnitus. Recommendation based on observational studies, with a preponderance of benefit over harm.
Action Statement Profile
- Quality improvement opportunity: To promote a consistent and systematic approach to the initial evaluation of the patient with tinnitus
- Aggregate evidence quality: Grade C, based on observational studies
- Level of confidence in evidence: Moderate, as few if any studies specifically investigate the diagnostic yield or impact of history and examination on tinnitus patients
- Benefits: Identify organic, and potentially treatable, underlying causes (e.g., secondary tinnitus); minimize cost and administrative burden through a targeted approach to history and physical examination; streamline care/increase efficiency; improve patient satisfaction; identify patients with primary tinnitus who may benefit from further management (as outlined in this guideline)
- Risks, harms, costs: None
- Benefit-harm assessment: Preponderance of benefit
- Value judgments: Perception by the GDG that tinnitus sufferers may not receive thorough evaluations from clinicians; further perception that many clinicians are unaware of the optimal targeted history and physical examination to evaluate a patient with tinnitus
- Intentional vagueness: The definition of a “targeted”history and physical examination is elaborated upon in the supporting text
- Role of Patient Preferences: None
- Exclusions: None
- Policy level: Recommendation
- Differences of opinion: None.
STATEMENT 2a. PROMPT AUDIOLOGIC EXAMINATION: Clinicians should obtain a comprehensive audiologic examination in patients with tinnitus that is unilateral, associated with hearing difficulties, or persistent (≥6months). Recommendation based on observational studies, with a preponderance of benefit over risk.
Action Statement Profile
- Quality improvement opportunity: To address potential underutilization of audiologic testing in patients with tinnitus who are likely to have underlying hearing loss and to avoid delay in such diagnosis
- Aggregate evidence quality:Grade C, based on observational studies
- Level of confidence in the evidence: Moderate, as literature about the impact of prompt audiologic assessment on tinnitus management is scant
- Benefits: Prioritize the need for otolaryngologic evaluation (if not already completed) using audiologic criteria; identify hearing loss, which is frequently associated with tinnitus; characterize the nature of hearing loss (conductive, sensorineural, or mixed; unilateral or bilateral); detect hearing loss that may be unsuspected; initiate workup for serious disease that causes unilateral tinnitus and hearing loss (i.e., VS)
- Risks, harms, costs: Direct cost of examination; access to testing; time
- Benefit-harm assessment: Preponderance of benefit
- Value judgments: None
- Intentional vagueness: The term “prompt”is used to emphasize the importance of ordering a timely test and ensuring it is done, preferably within four weeks of assessment
- Role of Patient Preferences: Small; patients may participate in decisions regarding timing of audiogram
- Exclusions: None
- Policy level: Recommendation
- Differences of opinion: None.
STATEMENT 2b. ROUTINE AUDIOLOGIC EXAMINATION: Clinicians may obtain an initial comprehensive audiologic examination in patients who present with tinnitus (regardless of laterality, duration, or perceived hearing status). Option based on observational studies, with a balance of benefit and harm.
Action Statement Profile
- Quality improvement opportunities: To promote awareness of hearing loss associated with tinnitus, even in patients who do not have unilateral tinnitus or hearing difficulties, and to emphasize that clinicians do not have to wait six months before obtaining an audiogram if deemed appropriate
- Aggregate evidence quality: Grade C, based on observational studies and prevalence of HL in RCTs of tinnitus therapy
- Level of confidence in the evidence: High
- Benefits:Detect a hearing loss not perceived by the patient; SNHL, which is a treatable condition commonly associated with tinnitus; identify patients who may be candidates for sound therapy; identify opportunities for patient counseling/education
- Risks, harms, costs: Direct costs of audiologic testing; detection of minor audiologic abnormalities leading to potentially unnecessary further testing or referral; inconsistent access to testing
- Benefit-harm assessment:Equilibrium
- Value judgments:None
- Intentional vagueness: None
- Role of patient preferences: Large role for shared-decision making to proceed with audiologic examination
- Exclusions: None
- Policy level: Option
- Differences of opinion: None.
STATEMENT 3. IMAGING STUDIES:Clinicians should not obtain imaging studies of the head and neck in patients with tinnitus, specifically to evaluate the tinnitus, unless they have one or more of the following: tinnitus that localizes to one ear, pulsatile tinnitus, focal neurological abnormalities, or asymmetric hearing loss. Strong recommendation against based on observational studies, with a preponderance of benefit over harm.
Action Statement Profile
- Quality improvement opportunity: Avoid overuse of imaging in patients with a low likelihood of any significant benefit from the imaging
- Aggregate evidence quality: Grade C, based on observational studies
- Level of confidence in the evidence: High
- Benefits: Avoid testing with low yield; avoid harms of unnecessary tests (radiation, contrast, cost); avoid test anxiety; avoid detecting subclinical, incidental findings
- Risks, harms, costs: Slight chance of missed diagnosis; relatively high costs and limited access to certain types of imaging studies
- Benefit-harm assessment: Preponderance of benefit
- Value judgments: GDG made this a strong recommendation against, instead of a recommendation against, based on consensus regarding the importance of avoiding low-yield, expensive tests with potential adverse events in patients with tinnitus
- Intentional vagueness: Specific imaging studies are specified in the supporting text including: CT, CTA, MRI, MRA
- Role of patient preferences: None
- Exclusions: None
- Policy level: Strong recommendation against
- Differences of opinion: None.
STATEMENT 4. BOTHERSOME TINNITUS: Clinicians must distinguish patients with bothersome tinnitus from patients with non-bothersome tinnitus. Strong recommendation based on inclusion criteria for RCTs on tinnitus treatment, with a preponderance of benefit over harm.
Action Statement Profile
- Quality improvement opportunity: To identify those patients in need of clinical management, and limit unnecessary testing and treatment for others
- Aggregate evidence quality: Grade B, based on inclusion criteria for RCTs on tinnitus treatment
- Level of confidence in evidence: High
- Benefits: Identify patients for further counseling and/or intervention/management; determine impact of tinnitus on health-related QOL; identify patients with bothersome tinnitus who may benefit from additional assessment for anxiety and depression; encourage an explicit and systematic assessment of patients to avoid underestimating or trivializing the impact of tinnitus; avoid unnecessary interventions/management of patients with non-bothersome tinnitus
- Risks, Harms, Costs: Time involved in assessment
- Benefit-Harm Assessment: Preponderance of benefit
- Value Judgments: None
- Intentional Vagueness: Method of distinguishing bothersome vs. non-bothersome is not specifically stated. One or more of the validated questionnaires described in the supporting text may be helpful
- Role of Patient Preferences: None
- Exclusions: None
- Policy Level: Strong recommendation
- Differences of opinion: None.
STATEMENT 5. PERSISTENT TINNITUS: Clinicians should distinguish patients with bothersome tinnitus of recent onset from those with persistent symptoms (≥6months) to prioritize intervention and facilitate discussions about natural history and follow-up care. Recommendation based on inclusion criteria in RCTs, with a preponderance of benefit over harm.
Action Statement Profile
- Quality improvement opportunity: To identify patients with a duration of tinnitus similar to that studied in RCTs of tinnitus treatment; to identify those who may need and benefit from intervention; and to avoid inappropriate interventions for patients with shorter duration tinnitus
- Aggregate evidence quality: Grade B, based on inclusion criteria in RCTs
- Level of confidence in the evidence: Moderate, based on varying tinnitus duration in RCTs, with some including patients with tinnitus of less than three months’duration
- Benefits: Identify patients who have duration of tinnitus similar to the patients included in RCTs, and identify those patients who are most likely to benefit from intervention
- Risks, Harms, Costs: Defer treatment that may benefit some tinnitus patients who do not have persistent symptoms
- Benefit-Harm Assessment: Preponderance of benefit
- Value Judgments: Despite some variation in inclusion criteria for duration of tinnitus used in clinical trials, the GDG felt that six months was a reasonable time to conclude that the tinnitus would likely persist
- Intentional Vagueness: None
- Role of Patient Preferences: None
- Exclusions: None
- Policy Level: Recommendation
- Differences of opinion: None.
STATEMENT 6. EDUCATION AND COUNSELING: Clinicians should educate patients with persistent, bothersome tinnitus about management strategies.Recommendation based on studies of the value of education and counseling, with a preponderance of benefit over harm.
Action Statement Profile
- Quality improvement opportunity: To address potential underutilization of education and counseling by clinicians who manage patients with persistent, bothersome tinnitus. To bring awareness of available management strategies to the patient
- Aggregate evidence quality: Grade B, based on studies of the value of education and counseling in general, and Grade C based on such studies in tinnitus in particular
- Level of confidence in the evidence: High
- Benefits: Improved QOL; increased ability to cope with tinnitus; improved outcomes and patient satisfaction; less healthcare utilization
- Risks, harms, costs: Direct cost and time
- Benefit-harm assessment: Preponderance of benefit
- Value judgments: None
- Intentional vagueness: None
- Role of patient preferences: None
- Exclusions: None
- Policy level: Recommendation
- Differences of opinion: None.
STATEMENT 7. HEARING AID EVALUATION: Clinicians should recommend a hearing aid evaluation for patients with hearing loss and persistent, bothersome tinnitus.Recommendation based on observational studies with a preponderance of benefit over harm.
Action Statement Profile
- Quality improvement opportunities: To promote awareness of the beneficial effect of hearing aids on tinnitus and encourage utilization of this first-line audiologic intervention for patients with tinnitus, even those who might otherwise be marginal hearing aid candidates
- Aggregate evidence quality: Grade C, based on observational studies
- Level of confidence in the evidence: High
- Benefits: Raise awareness of potential beneficial effects of hearing aids on tinnitus; ensure that patient receives proper guidance regarding benefits and costs of hearing aids; provide patients who have hearing loss with access to information and interventions that may alleviate hearing loss and improve function/QOL
- Risks, harms, costs: direct cost related to dispensing of a hearing aid
- Benefit-harm assessment: Preponderance of benefit
- Value judgments: Perceived lack of awareness regarding the ability of hearing aids to improve QOL for patients with tinnitus
- Intentional vagueness: The level of hearing loss is not specified because hearing loss-associated tinnitus may benefit from hearing aids even if the hearing loss is only of a mild degree, or even if there is a more severe unilateral SNHL associated with the tinnitus
- Role of patient preferences: Patient may accept or decline the recommendation to pursue a hearing aid evaluation
- Exclusions: None
- Policy level: Recommendation
- Differences of opinion: None.
STATEMENT 8. SOUND THERAPY: Clinicians may recommend sound therapy to patients with persistent, bothersome tinnitus. Option based on RCTs with methodological concerns, with a balance between benefit and harm.
Action Statement Profile
- Quality improvement opportunity: To promote awareness and utilization of sound therapy as a reasonable management option in patients with persistent, bothersome tinnitus
- Aggregate evidence quality: Grade B, based on RCTs with methodological concerns
- Level of confidence in the evidence: Medium, as strength of evidence is low.
- Benefits: Access to technology/devices that may relieve tinnitus; improve QOL, sleep, and concentration
- Risks, harms, costs: Consequences of recommending an intervention of uncertain efficacy; promoting false hope; costs associated with sound therapy
- Benefit-harm assessment: Equilibrium
- Value judgments: None
- Intentional vagueness: None
- Role of patient preferences: Significant role in deciding whether to pursue sound therapy and to choose among the available options
- Exclusions: None
- Policy level: Option
- Difference of opinion: One GDG member expressed a difference of opinion about mechanisms of sound therapy, in particular with the concepts of partial and total masking.
STATEMENT 9. COGNITIVE BEHAVIOR THERAPY (CBT): Clinicians should recommend CBT to patients with persistent, bothersome tinnitus. Recommendation based on RCTs, with a preponderance of benefit over harm.
Action Statement Profile
- Quality improvement opportunity: To promote awareness and utilization of CBT as an effective management option in patients with persistent, bothersome tinnitus
- Aggregate evidence quality: Grade A, based on multiple systematic reviews of RCTs
- Level of confidence in the evidence: Moderate, based on concerns about methodology and sample size of trials
- Benefits: Treatment of depression and anxiety; improved QOL, tinnitus coping skills, and adherence to other tinnitus treatments
- Risks, harms, costs: Direct cost; time involved (multiple sessions, 1-2 hrs. each); availability to services may be limited
- Benefit-harm assessment: Preponderance of benefit
- Value judgments: None
- Intentional vagueness: None
- Role of patient preferences: None
- Exclusions: None
- Policy level: Recommendation
- Differences in opinion: None.
 The guideline development process brings many perspectives to the table. This group (not the Tinnitus Guideline group) worked on such a guideline.
The guideline development process brings many perspectives to the table. This group (not the Tinnitus Guideline group) worked on such a guideline.STATEMENT 10. MEDICAL THERAPY: Clinicians should not routinely recommend antidepressants, anticonvulsants, anxiolytics, or intratympanic medications for a primary indication of treating persistent, bothersome tinnitus.Recommendation against based on systematic reviews and RCTs with methodological concerns, with a preponderance of benefit over harm.
Action Statement Profile
- Quality improvement opportunity: To decrease the use of medications that may have no benefit and have significant potential side effects, in the management of patients with tinnitus
- Aggregate evidence quality: Grade B, based on RCTs with methodological concerns and systematic reviews demonstrating a low strength of evidence
- Level of confidence in the evidence: Medium regarding the lack of efficacy of medical therapy as a primary treatment for persistent bothersome tinnitus, as several studies with methodological flaws, bias, and lack of power did show some benefit in certain tinnitus outcome measures
- Benefits: Avoid unproven therapy, side effects/adverse events (including tinnitus), and false hope; reduce expense. Avoid use of medications that are not approved for use in geriatric population
- Risks, harms, costs: Denying some patients benefit
- Benefit-harm assessment: Preponderance of benefit
- Value judgments: Although these therapies appear to be beneficial in some studies, the evidence from systematic reviews and RCTs is insufficient to justify routine use in managing tinnitus patients, especially given the known harms, cost of therapy, and potential for some medications (e.g., antidepressants) to worsen tinnitus
- Intentional vagueness: The term “routine”is used to acknowledge there may be individual circumstances for which clinicians and patients may wish to pursue therapy
- Role of patient preferences: Limited; a trial of medication may be administered based on individual circumstances
- Exclusions: Patients with depression, anxiety, or seizure disorders that constitute an indication for pharmacologic therapy independent of tinnitus
- Policy level: Recommendation against
- Differences in opinion: None.
STATEMENT 11. DIETARY SUPPLEMENTS: Clinicians should not recommend Ginkgo biloba, melatonin, zinc, or other dietary supplements for treating patients with persistent, bothersome tinnitus.Recommendation against based on RCTs and Systematic Reviews with methodological concerns, with a preponderance of benefit over harm.
Action Statement Profile
- Quality improvement opportunity: To avoid use of commonly-available supplements that have no proven efficacy and pose potential harm, in the management of patients with tinnitus
- Aggregate evidence quality: Grade C, RCTs and systematic reviews with extreme heterogeneity; most of the RCTs raise significant concerns regarding methodology and subject selection
- Level of confidence in the evidence: High confidence regarding potential harm and adverse effects related to these agents, particularly in the elderly population; low confidence in benefits due to methodological concerns and study quality and ability to generalize results to patients with persistent, primary tinnitus
- Benefits: Avoid unproven therapy, side effects/adverse events (including tinnitus), and false hope; reduce expense
- Risks, harms, costs: None
- Benefit-harm assessment: Preponderance of benefit
- Value judgments: Concern regarding the actual content and dosage of proposed active agents in these preparations, as they are currently packaged OTC. Many of these supplements, not under the regulations of the USFDA, have varying amounts of the “active”agent. The GDG was concerned over the widespread availability for easy purchase of these agents without considering potential drug interactions and adverse events
- Intentional vagueness: The term “dietary supplements”is used to generalize nutritional and herbal supplements promoted as remedies for tinnitus
- Role of patient preferences: Limited role
- Exclusions: None
- Policy level: Recommendation against
- Differences in opinion: The majority of the GDG felt there was a clear predominance of harm over benefit; a minority felt there was an equilibrium. None of the group perceived a preponderance of benefit over harm.
STATEMENT 12. ACUPUNCTURE: No recommendation can be made regarding the effect of acupuncture in patients with persistent bothersome tinnitus. No recommendation based on poor quality trials, no benefit, and minimal harm.
Action Statement Profile
- Quality improvement opportunity: Limited, to educate patients and providers about the controversies regarding the use of acupuncture for tinnitus
- Aggregate evidence quality: Grade C, based on inconclusive RCTs and the presence of costs and potential harm with no established benefit with the use of acupuncture for tinnitus
- Level of confidence in the evidence: Low regarding benefit because of heterogeneity and methodological flaws in the RCTs; high regarding harm or cost, with the understanding that serious harm from acupuncture is rare
- Benefits: No direct benefits of no recommendation
- Risks, harms, costs: Cost of acupuncture therapy, time required for therapy, and potential delay in instituting sound therapy or hearing aids
- Benefit-harm assessment: unknown
- Value judgments: The poor quality of the data, the limited potential for harm from acupuncture kept the GDG from making a recommendation about acupuncture
- Intentional vagueness: None
- Role of patient preferences: Significant role for shared decision making; patients may wish to try acupuncture based on circumstances
- Exclusions: None
- Policy level: No recommendation
- Differences in opinion: Minor: The GDG was divided between making no recommendation and making a recommendation against the use of acupuncture.
STATEMENT 13. TRANSCRANIAL MAGNETIC STIMULATION (TMS): Clinicians should not recommend TMS for the treatment of patients with persistent, bothersome tinnitus. Recommendation against based on inconclusive RCTs.
Action Statement Profile
- Quality improvement opportunity: To avoid use of a therapy that has inconclusive efficacy and poses potential financial and physical harm, in the management of patients with tinnitus
- Aggregate evidence quality: Grade B, based on inconclusive RCTs and systematic reviews that show low strength of evidence
- Level of confidence in the evidence: High regarding the absence of a long-term (>6 months) benefit of TMS; moderate regarding the absence of a short-term benefit, since a minority of trials demonstrated transient beneficial outcomes, and strength of this evidence is low
- Benefits: Avoid unproven therapy, side effects/adverse events, and false hope; reduce expense
- Risks, harms, costs: Denying some patients benefit
- Benefit-harm assessment: Preponderance of benefit
- Value judgments: None
- Intentional vagueness: None
- Role of patient preferences: Limited
- Exclusions: Patients with depression or other neurological conditions for which TMS is indicated
- Policy level: Recommendation against
- Differences in opinion: None.
Disclaimer
The clinical practice guideline is provided for information and educational purposes only. It is not intended as a sole source of guidance in managing patients with tinnitus. Rather, it is designed to assist clinicians by providing an evidence-based framework for decision-making strategies. The guideline is not intended to replace clinical judgment or establish a protocol for all individuals with this condition and may not provide the only appropriate approach to diagnosing and managing this program of care. As medical knowledge expands and technology advances, clinical indicators and guidelines are promoted as conditional and provisional proposals of what is recommended under specific conditions but are not absolute. Guidelines are not mandates; these do not and should not purport to be a legal standard of care. The responsible physician, in light of all circumstances presented by the individual patient, must determine the appropriate treatment. Adherence to these guidelines will not ensure successful patient outcomes in every situation. The AAO-HNSF emphasizes that these clinical guidelines should not be deemed to include all proper treatment decisions or methods of care, or to exclude other treatment decisions or methods of care reasonably directed to obtaining the same results.
References
Rosenfeld RM, Shiffman RN. Clinical Practice Guideline Development Manual: a quality-driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2009; 140(Suppl1). SI-43.
Hoffman HJ, Reed GW. Epidemiology of tinnitus. In: Snow JB. Ed. Tinnitus: Theory and Management. Lewiston, NY: BC Decker; 2004:16-41.
Henry JA, Zaugg TL, Myers PJ, Schechter MA. The role of audiologic evaluation in progressive audiologic tinnitus management. Trends Amplif. 2008;12(3):170-187.
Vital and Health Statistics: Current Estimates from the National Health Interview Survey, 1994. Series 10: Data from the National Health Survey No. 193; US Department of Health and Human Services Public Health Service, CDC, National Center for Health Statistics, DHHS Publication No. (PHS) 96-1521.
Nondahl DM, Cruickshanks KJ, Huang GH, et al. Tinnitus and its risk factors in the Beaver Dam Offspring Study. Int J Audiol. 2011; 50(5):313-320.
US Department of Veterans Affairs. Annual Benefits Report: Fiscal Year 2012. In: Affairs DoV, ed. Washington, DC. 2013.
Lewis JE, Stephens SDG, McKenna L. Tinnitus and suicide. Clin Otolaryngol Allied Sci. 1994;19(1):50-54.