Corneal Neurotization for Trigeminal Anesthesia
Trigeminal anesthesia may arise as a devastating consequence of extirpation of skull base tumors, such as large vestibular schwannomas. Insult to trigeminal nerve afferents leads to neurotrophic keratopathy (NK), a degenerative disease of the ocular surface characterized by progressive corneal epithelial defects, ulceration, vascularization, and perforation.
Nate Jowett, MD, and Roberto Pineda, MD
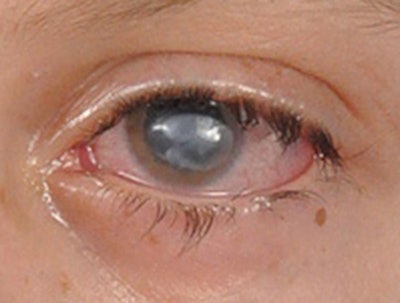 Figure 1. Neurotrophic keratitis. Severe corneal opacification is demonstrated in a patient with combined facial paralysis and trigeminal anesthesia following extirpation of a large vestibular schwannoma.
Figure 1. Neurotrophic keratitis. Severe corneal opacification is demonstrated in a patient with combined facial paralysis and trigeminal anesthesia following extirpation of a large vestibular schwannoma.Trigeminal anesthesia may arise as a devastating consequence of extirpation of skull base tumors, such as large vestibular schwannomas. Insult to trigeminal nerve afferents leads to neurotrophic keratopathy (NK), a degenerative disease of the ocular surface characterized by progressive corneal epithelial defects, ulceration, vascularization, and perforation. Paralytic lagophthalmos from concurrent facial nerve injury accelerates disease progression toward corneal blindness (Fig. 1).1
The pathophysiology of NK comprises absent protective sensory feedback, impaired blink and tear reflexes, and loss of neurotrophic support to the ocular surface. Neurotrophic factors, such as nerve growth factor, produced by and released from trigeminal nerve corneal afferents, are essential for the normal growth, differentiation, and survival of corneal cells.2,3 Even in the absence of mechanical trauma, loss of neurotrophic support to the ocular surface results in morphologic and metabolic disturbances that may impair vision.
Management of NK includes topical medical therapies, tarsorrhaphy, and bandage contact lenses. Though such interventions may halt NK progression and promote healing of the ocular surface, they do not address the underlying etiology of the disease.4 In 2009, it was demonstrated that NK progression could be reversed by transposition of healthy contralateral supraorbital and supratrochlear nerve branches to the affected ocular surface.5 Increasing evidence supports trigeminal sensory nerve transfer for vision protection and restoration in the setting of unilateral trigeminal anesthesia.6-8 Recently we demonstrated that transfer of sensory axons of the ipsilateral great auricular nerve to the corneal stroma using an interposition nerve autograft can improve vision and restore sensation to the ocular surface with minimal donor site morbidity (Fig. 2).9
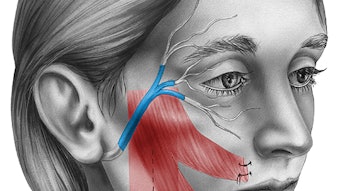
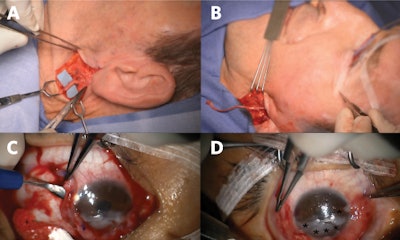 Figure 2. Great auricular nerve transfer for corneal neurotization. (A) Anterior and posterior branches of the great auricular nerve are identified. (B) A sural nerve autograft is harvested and inset between the great auricular nerve and inferior fornix. (C) Tunnels are made into the corneal stroma (C), and (D) nerve fascicles (*) positioned about the limbus to restore corneal sensory input.
Figure 2. Great auricular nerve transfer for corneal neurotization. (A) Anterior and posterior branches of the great auricular nerve are identified. (B) A sural nerve autograft is harvested and inset between the great auricular nerve and inferior fornix. (C) Tunnels are made into the corneal stroma (C), and (D) nerve fascicles (*) positioned about the limbus to restore corneal sensory input.Surgical transfer of healthy sensory axons to mitigate the loss of corneal afferent input is analogous to transfer of healthy motor axons of the hypoglossal nerve to restore facial tone in the setting of facial paralysis. As muscles demonstrate markedly decreased receptivity to neurotization following denervation periods exceeding one-two years, motor nerve transfers are contraindicated in the setting of long-standing facial paralysis. However, otolaryngologists and ophthalmologists need to be aware there exists no equivalent time limit when contemplating sensory neurotization of an anesthetic cornea; benefits have been reported for patients with histories of NK in excess of 20 years prior to corneal neurotization surgery.5 Corneal neurotization may carry particular benefit for patients with advanced cornea opacification or perforation, wherein the goal is to render them candidates for second-stage corneal transplant to help restore their vision.
Corneal neurotization is an emerging technique in the United States and the world and carries risk of iatrogenic vision loss. The most important factors determining success are the underlying disease process, surgeon experience, and each patient’s capacity to regenerate transferred nerves. In our opinion, the procedure is optimally performed in a team approach comprising specialists in corneal and cranial nerve surgery. We currently advise against the use of Avance® Nerve Grafts (Axogen, Inc.) for corneal neurotization, as considerable evidence points to increased risk of suboptimal neurotization where long processed nerve allografts are substituted for nerve autografts.10 Patients need to be aware that successful corneal neurotization does not guarantee improved vision and that corrective lenses may still be needed after surgery to maximize vision and protect the cornea. Though a successful surgical result decreases the risk of corneal injury and vision loss, some risk will remain. As corneal healing following neurotization in NK is believed to result from restoration of neurotrophic support to the ocular surface, future research should seek to compare the effectiveness of topical recombinant trophic factors alone (i.e., Cenegermin) or in combination with corneal neurotization procedures.
References:
- Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye 2003;17(8):989-95 doi: 10.1038/sj.eye.6700616[published Online First: Epub Date]|.
- Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Experimental eye research 2003;76(5):521-42
- F M. “De l’influence de la cinquieme paire de nerfs sur la nutrition et les fonctions de l’oeil. J Physiol Exp Pathol 1824;4:176-82
- Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clinical Ophthalmology (Auckland, N.Z.) 2014;8:571-79 doi: 10.2147/OPTH.S45921[published Online First: Epub Date]|.
- Terzis JK, Dryer MM, Bodner BI. Corneal neurotization: a novel solution to neurotrophic keratopathy. Plast Reconstr Surg 2009;123(1):112-20 doi: 10.1097/PRS.0b013e3181904d3a[published Online First: Epub Date]|.
- Bains RD, Elbaz U, Zuker RM, Ali A, Borschel GH. Corneal neurotization from the supratrochlear nerve with sural nerve grafts: a minimally invasive approach. Plast Reconstr Surg 2015;135(2):397e-400e doi: 10.1097/PRS.0000000000000994[published Online First: Epub Date]|.
- Catapano J, Fung SSM, Halliday W, et al. “Treatment of neurotrophic keratopathy with minimally invasive corneal neurotisation: long-term clinical outcomes and evidence of corneal reinnervation. British Journal of Ophthalmology 2019:bjophthalmol-2018-313042 doi: 10.1136/bjophthalmol-2018-313042[published Online First: Epub Date]|.
- Elbaz U, Bains R, Zuker RM, Borschel GH, Ali A. Restoration of corneal sensation with regional nerve transfers and nerve grafts: a new approach to a difficult problem. JAMA Ophthalmol 2014;132(11):1289-95 doi: 10.1001/jamaophthalmol.2014.2316[published Online First: Epub Date]|.
- Jowett N, Pineda Ii R. Corneal neurotisation by great auricular nerve transfer and scleral-corneal tunnel incisions for neurotrophic keratopathy. Br J Ophthalmol 2019;103(9):1235-38 doi: 10.1136/bjophthalmol-2018-312563[published Online First: Epub Date]|.
- Jowett N, Pineda Ii R. Acellular nerve allografts in corneal neurotisation: an inappropriate choice. Br J Ophthalmol 2020;104(2):149-50 doi: 10.1136/bjophthalmol-2019-315032[published Online First: Epub Date]|.