2018: Our focus now
As systemic changes progress, we are putting the finishing touches on the strategic plan and principles that will guide us through the upcoming uncertainty.
 James C. Denneny III, MD
James C. Denneny III, MD
AAO-HNS/F EVP/CEO
There was considerable discussion about the definition of what is a “private practitioner” as opposed to an “academician.” There is consensus that a continuum exists and that a clear demarcation of roles has blurred, particularly with the current status of employed physicians. Granted, there has been significant alteration of the predominant model for each group in the last 10 years. But in my experience, there continues to be nuanced differences that deserve recognition and individual consideration. My two stints in academic medicine and 25-plus years in private practice, along with extensive interaction with both groups during my time as EVP/CEO, confirm both the similarities and differences in the needs of each group and the strength that each brings to the Academy and the field of otolaryngology. We will continue to focus on the strengths as well as the needs of each group as we take the journey together.
OTC hearing aids: finalizing regulations
The Academy continues to work collaboratively with the FDA on several fronts critical to otolaryngologists and their patients. The process of finalizing regulations for the eventual over-the-counter (OTC) sale of hearing aids for patients 18 years of age or greater with a mild to moderate sensorineural hearing loss is moving forward. Along with key advocacy staff members, I had the opportunity to present the Academy’s concerns, suggestions, and support for the initiative. Our presentation centered on appropriate use, patient education and safety, and a process that will maximize the benefit of these critical medical devices. To increase the utilization and penetration of hearing aids in the marketplace, it is essential that patient experience and benefit are established from the outset. The potential opportunity this brings to the citizens of the United States is high if done correctly, but it also opens the door for potential misuse.
Sleep medicine conference upcoming
Arrangements are being finalized for the April 16, 2018, sleep medicine conference at FDA headquarters, of which we are a key participant. Kathleen Yaremchuk, MD, MSA, has agreed to lead our planning team as we work with the FDA and other organizations involved in the care of sleep disorders. We aim to construct a world-class program that includes advances in the field, but potentially more important, covers how we define and measure success of these innovations as well as existing treatments. It is imperative that otolaryngology be a leader in the diagnosis and treatment of sleep-disordered breathing in the evolution of management through innovation and in defining quality patient care. This conference can be a stepping stone for these goals.
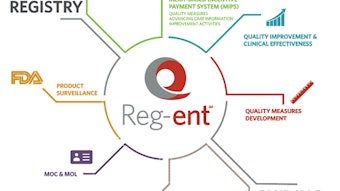
The Reg-entSM data registry
Our clinical data registry, Reg-ent, continues to grow in terms of the number of patient encounters and providers as well as capability. We continue to add measures across the breadth of the specialty and are working through problems with individual EHR vendors. We are reaching a critical mass that will allow us to continue work with the FDA to establish pre- and post-marketing protocols for both medical devices and pharmaceutical products. The opportunity to work with the FDA and industry in this fashion has great potential benefit for all parties, particularly in collecting consistent data across practice types and geographic locations, all of which benefit the patient.
Working for the specialty
I have received feedback from visiting many areas of the country and speaking with practitioners of all demographics, in addition to hearing the diverse input from strategic planning participants. This has made it clear to me that in the current environment, the Academy’s most important role is representing and advocating for the entire specialty on issues affecting otolaryngologists and their patients. Whether it involves legislation, regulation, private payer issues, maintenance of certification, physician wellness, or quality of care issues, we can only have a positive influence working together as a united house of otolaryngology. I want to thank all of you who have continued to be loyal members of the Academy and pledge to you our continued efforts on your behalf in these areas.