Perioperative Immunotherapy for Head and Neck Squamous Cell Carcinoma
Recent Phase III trials show that adding immunotherapy before and after surgery may significantly improve disease control for patients with advanced head and neck cancer.
Saahil Saxena, Kevin J. Contrera, MD, MPH, Katelyn O. Stepan, MD, and Janice L. Farlow, MD, PhD, on behalf of the Head and Neck Surgery Education Committee
The standard of care for other solid tumor types has gradually shifted toward neoadjuvant approaches or treatment prior to definitive intervention. Neoadjuvant treatment has the potential to reduce tumor burden, decrease the extent of definitive treatment required, address possible early metastatic disease, and/or enhance long-term survival. The American Head and Neck Society recently released a narrative review summarizing the current state of research on neoadjuvant therapy for mucosal HNSCC, highlighting its promise based on Phase II data.² The review outlines options for chemotherapy, immunotherapy, radiation, or combinatorial treatment. This approach can potentially benefit patients through tumor downstaging, improved surgical outcomes, and eradication of micrometastatic disease.
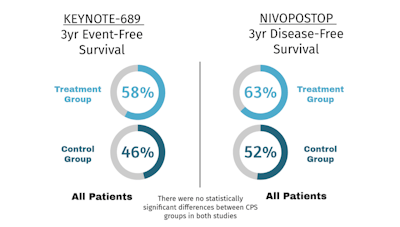
Recently, preliminary results were presented from the randomized, multinational Phase III KEYNOTE-689 trial.³⁻⁵ In this trial, pembrolizumab, a programmed cell death protein 1 receptor antagonist, was given before and after surgery for resectable, locoregionally advanced HNSCC. The study examined 714 patients (stage III/IVA oral cavity, larynx, hypopharynx, p16⁻ oropharynx; or stage III p16⁺ oropharynx). The control arm received standard surgery with pathology-guided adjuvant treatment, while the experimental arm had standard of care plus two doses of pembrolizumab prior to surgery, and pembrolizumab in the adjuvant and maintenance setting. At the first interim analysis (36 months), results showed statistically significant improvement in event-free survival and major pathologic response in the experimental group. The major survival signal appeared to be driven by improved distant metastasis-free survival (HR 0.71, 95% CI 0.56-0.90). Adverse events were consistent with those associated with (chemo)radiation and were similar between treatment groups.
Also presented at the 2025 American Society of Clinical Oncology Annual Meeting were the preliminary results from NIVOPOSTOP.⁶ In this Phase III randomized trial, anti-PD1 nivolumab versus placebo was given in the adjuvant setting with standard pathology-guided adjuvant radiotherapy with or without chemotherapy for locoregionally advanced HNSCC (stage III/IVA oral cavity, oropharynx, larynx, or hypopharynx). A total of 680 patients were randomized. Disease-free survival was significantly better when nivolumab was added to standard adjuvant treatment (HR 0.76, 95% CI 0.60-0.98) at 36 months. Further analysis suggested that improved oncologic outcomes were driven by improved locoregional control. Adverse events again were most consistent with those associated with (chemo)radiation, with a slight increase in late treatment-related adverse events in the experimental arm.
Integrating perioperative immunotherapy for locoregionally advanced HNSCC is a promising approach. Insights from recent studies suggest that this approach may enhance disease control, although data on whether this actually improves overall survival are still immature. Application of this strategy to patients outside of the strict study inclusion criteria is unknown. Furthermore, surgeons must be able to counsel and monitor patients undergoing neoadjuvant treatment for disease progression and adverse events from immunotherapy.
More work must be done to understand long-term survival benefits, sequencing and duration of treatments, predictive biomarkers, and best practices for multidisciplinary coordination and ensuring equity in access.
References
- Barsouk A, Aluru JS, Rawla P, Saginala K, Barsouk A. "Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma." Medical Sciences 11, no. 2 (June 13, 2023): 42. https://doi.org/10.3390/medsci11020042.
- Contrera KJ, Kansara S, Goyal N, et al. "Neoadjuvant Therapy for Mucosal Head and Neck Squamous Cell Carcinoma: A Review From the American Head and Neck Society." JAMA Otolaryngology–Head & Neck Surgery, published online April 24, 2025. https://doi.org/10.1001/jamaoto.2025.0410.
- Uppaluri R, et al. "2025 AACR Annual Meeting." Abstract CT001. Presented April 27, 2025.
- Uppaluri R, et al. "KEYNOTE-689: Phase 3 Study of Adjuvant and Neoadjuvant Pembrolizumab Combined with Standard of Care (SOC) in Patients with Resectable, Locally Advanced Head and Neck Squamous Cell Carcinoma." Journal of Clinical Oncology 37 (2019): TPS6090-TPS6090. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS6090.
- Adkins D, et al. "2025 ASCO Annual Meeting." Abstract 6012. Presented June 1, 2025.
- Bourhis J, et al. "2025 ASCO Annual Meeting." Abstract LBA2. Presented June 1, 2025.