Vestibular Migraines and Vestibular Therapy
Vestibular migraine (VM) has become an increasingly recognized etiology of episodic vertigo. However, due to its overlapping symptomatology with peripheral vestibular disorders and a lack of awareness in the medical community, VM is frequently underdiagnosed or mismanaged. The diagnostic criteria for VM are based on the International Classification of Headache Disorders and the International Classification of Vestibular Disorders (ICVD) of The Bárány Society.
Misheelt Batjargal, MD, Varun V. Varadarajan, MD, and Oliver F. Adunka, MD
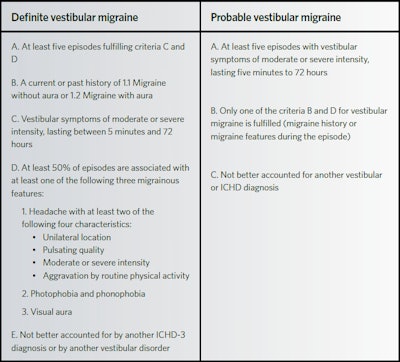
Vestibular migraine (VM) has become an increasingly recognized etiology of episodic vertigo.1-5,7 However, due to its overlapping symptomatology with peripheral vestibular disorders and a lack of awareness in the medical community, VM is frequently underdiagnosed or mismanaged.3 The diagnostic criteria for VM are based on the International Classification of Headache Disorders and the International Classification of Vestibular Disorders (ICVD) of The Bárány Society (Table 1).1,3,5,6,11-14 The Bárány Society criteria classifies the diagnosis as either “actual” VM or “probable” VM.3,5,9,12
The association of VM with audiologic symptoms may mislead the clinician and subsequently lead to a particularly challenging diagnostic process. To add to the confusion, there is an increased incidence of cochlear disorders in migraine patients.8 Pure tone audiometry may be affected to a mild degree, particularly in the low frequencies. A symmetric and nonprogressive pattern has been described by several authors.3,5,7 Central auditory pathways also appear to be affected in some individuals, with a prolonged wave V peak and interpeak latencies reported on auditory brainstem response.3,7 In up to two-thirds of affected individuals, VM may closely resemble Ménière’s disease (MD) with accompanying tinnitus, aural fullness, fluctuating low-frequency sensorineural hearing loss (SNHL), or sudden SNHL.5,7,8 Conversely, migraines are more commonly reported in patients with MD, and patients may meet diagnostic criteria for both conditions.5,8 Cervical and ocular vestibular-evoked myogenic potentials (VEMPs) have shown conflicting results in VM patients, and some reports shows no difference between patients with migraine, VM, and healthy controls.3,5
Identifying triggers may aid in the diagnosis of VM when the history does not suggest a clear etiology. Common triggers for VM attacks are stress, bright lights, sleep deprivation, weather (barometric pressure) changes, physical motion, visual triggers, noisy environments, and dietary tyramine such as aged cheese, cured meat, and alcohol.6 There has been an increased prevalence of sleep disorders and poor sleep quality in VM patients.5,6 Psychiatric comorbidities, especially anxiety and depression, are also common in patients with VM and are associated with an increased incidence of migraine disorders.3 Some patients with VM may experience Alice in Wonderland syndrome, characterized by dysperceptions between episodes that may be mistaken for psychosis.3
Trigger avoidance, the avoidance of high tyramine and caffeine foods, proper sleep hygiene, regular meals, exercise, and stress management are considered by many to be the first line treatment for suspected VM. Diet and lifestyle modifications alone may be effective in some patients.3,5 Pharmacologic treatment may be classified into abortive treatment as well as preventative management. Benzodiazepines, meclizine, dimenhydrinate, or transdermal scopolamine could be offered for abortive treatment during severe attacks. Newer serotonin receptors antagonist (triptan derivatives),1 venlafaxine, and valproic acid are effective in early phase of the VM attack and overall dizziness.3 Prophylactic treatments are indicated when VM attacks occur for several months, continue over several weeks, or if the patient’s lifestyle is severely impacted. Antihypertensives, antidepressant, and antiepileptic drugs are often effective in reducing the frequency and severity of VM symptoms.5 A stepwise approach may start with beta-blocker or calcium-channel blocker (Propranolol 40-240 mg, Metoprolol 50-200 mg, diltiazem 120-240 mg, nimodipine 30-90mg).1 Flunarizine and cinnarizine are alternative calcium channel blockers that may decrease the severity or frequency of vertigo.3,5,9 Tricyclic antidepressants and carbonic anhydrase inhibitors (acetazolamide) have been reported to demonstrate similar efficacy in symptom reduction.5,9 Surgical intervention remains controversial: neuromodulation with vagal nerve stimulation or external trigeminal nerve stimulation have been described as potentially effective rescue treatments.2,4 However, prospective clinical trials are warranted before surgical intervention is routinely recommended for VM.10
Vestibular rehabilitation (VR) may be offered as an adjunct in the treatment of chronic balance dysfunction in VM. VR is based on central mechanisms of neuroplasticity, which includes adaptation, habituation, and substitution that facilitate vestibular compensation.10,11 VR aims to reduce functional impairment by targeting gaze stability, habituation, and enhancing gait and overall balance. Some other components of VR are general strengthening and stretching exercises, balance retraining exercises, aerobic training, swimming or cycling, and vestibulo-ocular and vestibulo-spinal reflex training.11 The efficacy of VR has yet to be confirmed via larger, prospective studies; however, a recent systematic review reported that all patients benefitted to some degree from a customized VR program.10 Outcome measures such as the Activities-Specific Balance Confidence Scale, Dizziness Handicap Inventory, and/or the Perception of Dizziness Symptoms tool all demonstrated significant improvement in both VM patients as well as nonmigrainous vestibular disorder group.10
References
- Lapira A. (2019). Vestibular migraine treatment and prevention. Behandlung und Prävention von vestibulärer Migräne. HNO, 67(6), 425–428. https://doi.org/10.1007/s00106-019-0661-3
- Beh, S. C., & Friedman, D. I. (2019). Acute vestibular migraine treatment with noninvasive vagus nerve stimulation. Neurology, 93(18), e1715–e1719. https://doi.org/10.1212/WNL.0000000000008388
- Beh S. C. (2019). Vestibular Migraine: How to Sort it Out and What to Do About it. Journal of Neuro-Ophthalmology, 39(2), 208–219. https://doi.org/10.1097/WNO.0000000000000791
- Beh S. C. (2020). External trigeminal nerve stimulation: Potential rescue treatment for acute vestibular migraine. Journal of the Neurological Sciences, 408, 116550. https://doi.org/10.1016/j.jns.2019.116550
- Huang, T. C., Wang, S. J., & Kheradmand, A. (2020). Vestibular migraine: An update on current understanding and future directions. Cephalalgia, 40(1), 107–121. https://doi.org/10.1177/0333102419869317
- Beh, S. C., Masrour, S., Smith, S. V., & Friedman rescue, D. I. (2019). The Spectrum of Vestibular Migraine: Clinical Features, Triggers, and Examination Findings. Headache, 59(5), 727–740. https://doi.org/10.1111/head.13484
- Xue, J., Ma, X., Lin, Y., Shan, H., & Yu, L. (2020). Audiological Findings in Patients with Vestibular Migraine and Migraine: History of Migraine May Be a Cause of Low-Tone Sudden Sensorineural Hearing Loss. Audiology & Neuro-otology, 1–6. Advance online publication. https://doi.org/10.1159/000506147
- Hwang, J. H., Tsai, S. J., Liu, T. C., Chen, Y. C., & Lai, J. T. (2018). Association of Tinnitus and Other Cochlear Disorders With a History of Migraines. JAMA Otolaryngology – Head & Neck Surgery, 144(8), 712–717. https://doi.org/10.1001/jamaoto.2018.0939
- Domínguez-Durán, E., Montilla-Ibáñez, M. A., Álvarez-Morujo de Sande, M. G., Domènech-Vadillo, E., Bécares-Martínez, C., González-Aguado, R., & Guerra-Jiménez, G. (2020). Analysis of the effectiveness of the prophylaxis of vestibular migraine depending on the diagnostic category and the prescribed drug. European Archives of Oto-Rhino-Laryngology, 277(4), 1013–1021. https://doi.org/10.1007/s00405-020-05802-5
- Kinne, B.L., Baker, B.J., Chesser, B.T (2017). The use of Vestibular Rehabilitation for Individuals with Migraines: A Systematic Review. Otolaryngol (Sunnyvale) 7: 334. Doi:10.4172/2161-119X.1000334
- Alghadir, A. H., & Anwer, S. (2018). Effects of Vestibular Rehabilitation in the Management of a Vestibular Migraine: A Review. Frontiers in Neurology, 9, 440. https://doi.org/10.3389/fneur.2018.00440
- Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J, et al. Vestibular migraine: Diagnostic criteria. J. Vestib. Res. Equilib. Orientat. 2012;22:167–72.
- Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56:436–41.
- Radtke A, Neuhauser H, von Brevern M, Hottenrott T, Lempert T. Vestibular migraine–validity of clinical diagnostic criteria. Cephalalgia. 2011;31:906–13.