Annual Report 2015: Research & Quality
You have asked us to build a sustainable infrastructure for research and quality products to promote translational research and evidence-based medicine. You asked that we demonstrate the value of research and administer the granting program for the specialty. We Listened and acted with these accomplishments. You asked that we demonstrate the value of research and administer the granting program for the specialty. We Listened and acted with these accomplishments.
YOU SPOKE. WE LISTENED: RESEARCH & QUALITY
 2015 Miniseminar: The Power of Data: Creating a Clinical Data Registry for Otolaryngology—Head and Neck Surgery
2015 Miniseminar: The Power of Data: Creating a Clinical Data Registry for Otolaryngology—Head and Neck SurgeryYou have asked us to build a sustainable infrastructure for research and quality products to promote translational research and evidence-based medicine. You asked that we demonstrate the value of research and administer the granting program for the specialty. We Listened and acted with these accomplishments:
- Approved: The clinical data registry initiative was approved by the Board in March 2015.
- Named and Branded Regent℠: The AAO-HNSF ENT clinical data registry name and branding–Regent℠ was approved by the Executive Committee and a registry vendor, FIGmd, was approved by the Executive Committee in August and the full Board in September 2015.
- Endorsed: Two Acute Otitis Externa (AOE) and three Otitis Media with Effusion (OME) measures were submitted for National Quality Forum (NQF) endorsement. All submitted measures received full endorsement, with the exception of OME; Systemic Use of Corticosteroids. This measure will be endorsed with a reserve status due to a small performance gap and limited opportunity for improvement.
- Published: The PSQI Committee completed a manuscript entitled “Quality in Otolaryngology: Where We Have Been and Where We Are” which was published in the May 2015 journal.
- Published: Clinical Practice Guidelines (CPG): Allergic Rhinitis was published February 2015; CPG: Adult Sinusitis update published April 2015; the Clinical Consensus Statement (CCS) Development Manual was published November 2015 and the CCS: Septoplasty was published November 2015. CPG updates in process include: Otitis Media with Effusion; Cerumen Impaction; BPPV; Hoarseness; and two new products including CPG: Evaluation of the Neck Mass in Adults and CPG: Rhinoplasty.
- Viewed and Cited: The AAO-HNSF CPGs have been viewed almost 530,000 times via the National Guideline Clearinghouse and the CPGs/CCSs/Manuals have been cited almost 3,400 times, as reported by Google Scholar.
- Awarded: The 2015 CORE grant program participating societies (AAO-HNSF, AHNS, ASPO, ARS, AAFPRS) awarded 35 grants totaling $488,000. Of that amount, AAO-HNSF and its sponsors (Alcon, Cook Medical, and Oticon) funded 28 grants totaling $278,750.
CLINICAL PRACTICE GUIDELINES (CPG) & CLINICAL CONSENSUS STATEMENTS (CCS)
Members have asked for more guidelines and more derivative products. We are producing more CPGs and CCSs than ever before and AAO-HNSF now makes slide sets available for education, pocket cards for easy bedside reference, and patient educational materials to facilitate shared decision-making.

Six additional guideline products are in various stages of development:
- CPG Otitis Media with Effusion: To publish early 2016
- CPG Cerumen Impaction: Under external peer review
- CPG Benign Paroxysmal Positional Vertigo: Undergoing update
- CPG Hoarseness: Undergoing update

- CPG Rhinoplasty: Under development
- CPG Evaluation of the Neck Mass in Adults: Under development
AAO-HNSF guidelines have been accessed over half a million times via the National Guideline Clearinghouse and citations of the CPGs are over 3,000. We have received almost 80 requests for the CPG slide sets from both Members and non-members across the world, resulting in an estimated 147 presentations of the AAO-HNSF guidelines to various stakeholders.
AAO-HNSF continues to collaborate with the Creating Healthcare Excellence through Education and Research (CHEER) Practice-based Research network to research adherence to the CPGs and barriers to implementing the guidelines. Three studies looking at the Voice Outcomes after Thyroid Surgery, Sudden Hearing Loss, and Tympanostomy Tubes are nearing completion. These study findings will help us to inform future performance measures development and additional resources needed by our Members to implement the guidelines for the best care to the patients they serve.
All new guidelines and consensus statement products were presented as Miniseminars at the AAO-HNSF 2015 Annual Meeting & OTO EXPO℠ along with an Instruction Course on Understanding Clinical Practice Guidelines. Online Lecture Series were recorded for Adult Sinusitis (Update) and Otitis Media with Effusion (Update) and will be available to the members in early 2016.
For many years, there has been interest in translating the CPGs. This year we entered into a project with the Mexican ORL Society (SMORL) to “ADAPTE” several of the AAO-HNSF CPGs to the Mexican community.
We continue to develop harmonization pieces that compare and contrast our guidelines to other organizations. Two products are in development: AAO-HNSF Adult Sinusitis CPG compared to the IDSA CPG; and AAO-HNSF Allergic Rhinitis CPG with the AAAAI/ACAAI practice parameters.
CENTRALIZED OTOLARYNGOLOGY RESEARCH EFFORTS (CORE) GRANT PROGRAM
AAO-HNSF has entered into a new partnering strategy with participating specialty societies to administer the CORE grant program, which will allow for more funds to be available for research.
The CORE grant program received 162 research applications (18 resubmissions) from 71 institutions. In total, applicants were requesting $2,539,225 in funding. The 2015 CORE leadership (including the boards and councils of all participating societies) approved a portfolio of 35 grants totaling $519,006. Of that amount, AAO-HNSF funded 28 grants totaling $278,750.
YOU SPOKE. WE LISTENED: REGENT℠
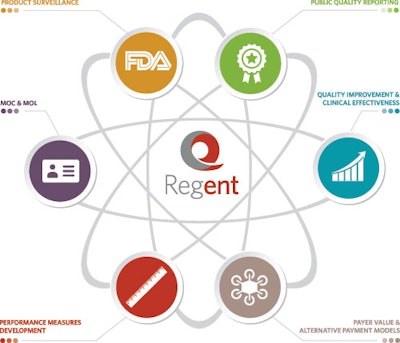
Members have asked for a clinical data registry. We Listened and are building Regent℠, the first ENT clinical data registry to: facilitate new measures development for the depth and breadth of the specialty, help Members demonstrate the value of care they provide, enable quality reporting and improvement, address clinical effectiveness, facilitate Maintenance of Certification and Licensure, and enable product surveillance.

WHAT IS REGENT℠?

ACCOMPLISHMENTS
- Completed an environmental scan with other medical and surgical specialties with registries; and
- Validated the clinical data registry approach with CMS, private payers and the Large Group Forum;
- Completed a Request for Information (RFI) with vendor finalists and held a registry vendor educational summit in June; held regular Task Force calls to ensure alignment.
- Finalized the registry strategy, name (Regent℠) and logo;
- Completed the registry business plan, RFP, registry vendor assessment and identified FIGmd as the top vendor; and
- Received approval of FIGmd from the Executive Committee and full Boards.
- A Regent℠ Miniseminar entitled, “The Power of Data: Creating a Clinical Data Registry for Otolaryngology” was held at Annual Meeting in Dallas, TX.
- Regent℠ Booth at Annual Meeting hosted demonstrations and presentations.
There was significant interest in Regent℠ among Members who visited the booth and a common theme emerged in their questions. We compiled the top three and answer them below.
WHAT WILL REGENT℠ OFFER?

Regent℠ will help Members report on quality measures for federal and private programs.
- Demonstration of Value and Alternative Payment Models
Regent℠ will affirm effectiveness and quality of care provided; utilize data to more effectively negotiate with payers; and prepare for participation in alternative payment models.
- Quality Improvement and Clinical Effectiveness
Regent℠ will utilize longitudinal data to identify gaps in performance and gaps in care provided both at the individual practitioner and system levels.
- Maintenance of Certification and Licensure
Regent℠ will coordinate with and provide data to the American Board of Otolaryngology (ABOto) and state licensing boards to assist in satisfying MOC and MOL requirements.
- Performance Measure Development
Regent℠ will allow more rapid and cost-effective development of measures to rapidly expand our portfolio through a measure authoring tool. This will allow the AAO-HNSF to author and simulate measures on registry data before launching the measures.
- Product Surveillance
Regent℠ will facilitate post-market surveillance activities.
WHAT’S NEXT FOR REGENT℠?

WHAT’S NEXT FOR PERFORMANCE MEASURES?
Now that the AAO-HNSF has secured National Quality Forum Endorsement for the acute otitis externa and otitis media with effusion measures, the Performance Measures Task Force will be establishing its performance measure methodology and developing new measures for allergic rhinitis and BPPV. We will be looking to the specialty societies to assist in development of new measures. A new area for measure development with Regent℠ includes outcome measures development, including patient reported outcomes.
To stay up to date visit www.entnet.org/Regent
Have questions? Email Regent@entnet.org