Elements from the New or Revised CPT Code Application
AAO-HNS Physician Payment Policy Group (3P) Name of applying party. Name and brief description of service/procedure. Reason for application/background information. Is this a revision of an existing code, request for revaluation of an existing code, request for a new code, or inquiry regarding proper coding for new technology? Is the service/procedure FDA-approved for the specific use of applicable devices or drugs? Is the service/procedure performed by many physicians/practitioners across the United States? If not widely practiced, provide names of individuals/centers providing this service. Is the service/procedure currently being reported by one or more existing codes? If so, which codes are being used? If a new code request, is this for Category I or III? For Category III code requests, please attach the following: A protocol of the study or procedures being performed. Please attach, along with descriptions of current U.S. trials outlining efficacy of the procedure. Support from the specialty societies who would use this procedure. Availability of U.S. peer-reviewed literature for examination by the CPT Editorial Panel. Please supply electronic copies of any available references and fill in reference grid below, assigning levels of evidence using the table provided. Descriptions of current U.S. trials outlining the efficacy of the procedure. For Category I code requests (new or revised), is the clinical efficacy of the service/procedure well-established and documented in U.S. peer-reviewed literature? If so, please supply electronic copies of references and fill in the reference grid below. Optimally, five references should be submitted, of which at least three report the procedure/service in U.S. patient populations. At least two articles should report different patient populations or have different, non-overlapping authors. Foreign references are acceptable if published in English and relevant or applicable to U.S. populations. Please assign level of evidence for each reference from the table below. Note that, for codes describing new procedures, at least one publication should meet or exceed the criteria for level III. Are there subspecialty societies within our specialty that are supporting this application? If so, please list, including contact information. Are there subspecialty committees within AAO-HNS that are supporting this application? If so, please list, including contact information. Are there members of other specialties that may also perform this procedure/service? If so, please list. After the Health Policy department receives an application, it is reviewed to ensure completeness. Next, the application is routed to 3P, which will evaluate the request and take appropriate action, with input from Academy committees that relate to the specific procedure or service. The Medical Devices and Drugs Committee will assess the safety, efficacy, and adoption of any new technology. During the process, the applicant will receive communications from Health Policy and 3P representatives. The Academy Board will also receive regular updates from these sources. If you are interested in submitting a code change proposal to the CPT Editorial Panel, you must follow this process in order for the Academy to support the proposal. Make sure to submit the New or Revised CPT Code Application to the Academy at least six to eight weeks before the code change proposal submission date to the American Medical Association so there is time for thorough review and approval. For more information, visit (http://www.entnet.org/Practice/Applying-for-CPT-codes-and-Obtaining-RVU.cfm) or email Jenna Kappel, director of Health Policy, at JKappel@entnet.org. Level Type of evidence (based on AHCPR 1992) Ia Evidence obtained from meta-analysis of randomized controlled trials Ib Evidence obtained from at least one randomized controlled trial IIa Evidence obtained from at least one well-designed controlled study without randomization IIb Evidence obtained from at least one other type of well-designed quasi-experimental study III Evidence obtained from well-designed non-experimental descriptive studies, such as comparative studies, correlation studies, and case control studies IV Evidence obtained from expert committee reports or opinions and/or clinical experience of respected authorities V Evidence obtained from case reports or case series References Level of Evidence Based on LOE Table U.S. or Foreign Peer Reviewed U.S. or Foreign Population Studied Prospective Study Total Patients Studied Article (Author, Title, Journal, Year, Volume, and Pages) Insert level # U.S. Foreign U.S. Foreign Both Yes No Insert # Provide brief description regarding relevance to the CPT process. * For each article cited, please provide a brief description of why the specific literature reference is relevant (e.g. “this is the hallmark double-blinded controlled study establishing the value of the procedure/service,” “this is a case report describing the procedure/service in detail,” or “this is an opinion statement from a respected authority in the field”).
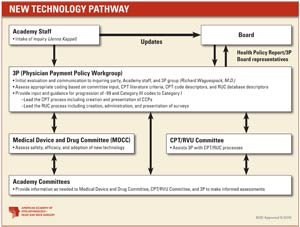
Physician Payment Policy Group (3P)
- Name of applying party.
- Name and brief description of service/procedure.
- Reason for application/background information.
- Is this a revision of an existing code, request for revaluation of an existing code, request for a new code, or inquiry regarding proper coding for new technology?
- Is the service/procedure FDA-approved for the specific use of applicable devices or drugs?
- Is the service/procedure performed by many physicians/practitioners across the United States? If not widely practiced, provide names of individuals/centers providing this service.
- Is the service/procedure currently being reported by one or more existing codes? If so, which codes are being used?
- If a new code request, is this for Category I or III?
- For Category III code requests, please attach the following:
- A protocol of the study or procedures being performed. Please attach, along with descriptions of current U.S. trials outlining efficacy of the procedure.
- Support from the specialty societies who would use this procedure.
- Availability of U.S. peer-reviewed literature for examination by the CPT Editorial Panel. Please supply electronic copies of any available references and fill in reference grid below, assigning levels of evidence using the table provided.
- Descriptions of current U.S. trials outlining the efficacy of the procedure.
- For Category I code requests (new or revised), is the clinical efficacy of the service/procedure well-established and documented in U.S. peer-reviewed literature? If so, please supply electronic copies of references and fill in the reference grid below. Optimally, five references should be submitted, of which at least three report the procedure/service in U.S. patient populations. At least two articles should report different patient populations or have different, non-overlapping authors. Foreign references are acceptable if published in English and relevant or applicable to U.S. populations. Please assign level of evidence for each reference from the table below. Note that, for codes describing new procedures, at least one publication should meet or exceed the criteria for level III.
- Are there subspecialty societies within our specialty that are supporting this application? If so, please list, including contact information.
- Are there subspecialty committees within AAO-HNS that are supporting this application? If so, please list, including contact information.
- Are there members of other specialties that may also perform this procedure/service? If so, please list.
After the Health Policy department receives an application, it is reviewed to ensure completeness. Next, the application is routed to 3P, which will evaluate the request and take appropriate action, with input from Academy committees that relate to the specific procedure or service. The Medical Devices and Drugs Committee will assess the safety, efficacy, and adoption of any new technology. During the process, the applicant will receive communications from Health Policy and 3P representatives. The Academy Board will also receive regular updates from these sources. If you are interested in submitting a code change proposal to the CPT Editorial Panel, you must follow this process in order for the Academy to support the proposal. Make sure to submit the New or Revised CPT Code Application to the Academy at least six to eight weeks before the code change proposal submission date to the American Medical Association so there is time for thorough review and approval.
For more information, visit (http://www.entnet.org/Practice/Applying-for-CPT-codes-and-Obtaining-RVU.cfm) or email Jenna Kappel, director of Health Policy, at JKappel@entnet.org.
| Level | Type of evidence (based on AHCPR 1992) |
|---|---|
| Ia | Evidence obtained from meta-analysis of randomized controlled trials |
| Ib | Evidence obtained from at least one randomized controlled trial |
| IIa | Evidence obtained from at least one well-designed controlled study without randomization |
| IIb | Evidence obtained from at least one other type of well-designed quasi-experimental study |
| III | Evidence obtained from well-designed non-experimental descriptive studies, such as comparative studies, correlation studies, and case control studies |
| IV | Evidence obtained from expert committee reports or opinions and/or clinical experience of respected authorities |
| V | Evidence obtained from case reports or case series |
| References | Level of Evidence Based on LOE Table | U.S. or Foreign Peer Reviewed | U.S. or Foreign Population Studied | Prospective Study | Total Patients Studied |
|---|---|---|---|---|---|
| Article (Author, Title, Journal, Year, Volume, and Pages) | Insert level # | U.S. Foreign | U.S. Foreign Both | Yes No |
Insert # |
| Provide brief description regarding relevance to the CPT process. | |||||
| * For each article cited, please provide a brief description of why the specific literature reference is relevant (e.g. “this is the hallmark double-blinded controlled study establishing the value of the procedure/service,” “this is a case report describing the procedure/service in detail,” or “this is an opinion statement from a respected authority in the field”). | |||||