Research and Quality Improvement Accomplishments
As you can tell from reading through these pages, there is an abundance of work being done on behalf of our members in the areas of quality and research. It is imperative, given all of the focus from external organizations including the government, private payers, and certifying boards (ABOto and the umbrella ABMS organization), that we are involved in initiatives that demonstrate the specialty’s continued focus on improving performance in practice. I want to highlight several of these initiatives in this column. The Patient Safety and Quality Improvement (PSQI) Committee (led by David W. Roberson, MD, and Rahul K. Shah, MD) agreed to spearhead the AAO-HNS Foundation’s participation with the American Board of Internal Medicine (ABIM) Foundation’s Choosing Wisely® campaign last year. The main focus of the campaign was to identify tests and/or procedures that should be questioned and to engage patients in discussions about appropriateness of care. This was a great example of the specialties working together toward a common goal. The PSQI engaged other academy and foundation committees, the Specialty Society Advisory Council (SSAC) and the Guideline Task Force (GTF), in the development of an AAO-HNS list, and many of the suggestions came from AAO-HNS clinical practice guideline action statements. (Further information on Choosing Wisely appears in the PSQI summary on p. 32.) As the first surgical specialty to join the campaign, we were invited to participate in the Washington press conference in February at the Kaiser Family Foundation headquarters to roll out our list with other specialty societies. A commentary article on the campaign appeared in the April 2013 edition of Otolaryngology–Head and Neck Surgery. The PSQI committee was also instrumental in working with the FDA on our members’ behalf when the FDA contacted us about its plans to issue a directive regarding the use of codeine post tonsillectomy and/or adenoidectomy. The PSQI Committee proactively emailed members once it was informed that the FDA was moving in this direction. The committee then kept in touch with the FDA as it came out with an alert in August warning of the risk of possible fatality when codeine is used in this clinical setting and when it ultimately issued a black box warning and contraindication in February. The FDA agreed to co-author a commentary with PSQI Committee co-chair, Dr. Roberson, and our Executive Vice President and CEO, David R. Nielsen, MD, which was published in the New England Journal of Medicine (http://bit.ly/NEJMdrug). This year, Research and Quality Improvement has partnered even more effectively with the Physician Payment Policy (3P) Workgroup to address many of the tenets of healthcare reform relating to quality, including public reporting of physician data initiatives, Medicare incentive programs, and alternative payment models. In April, physician leaders from the 3P Workgroup and the PSQI Committee, along with staff from both business units, met with officials at the Centers for Medicare & Medicaid Services (CMS) to receive feedback and discuss not only the Academy’s significant accomplishments in the area of quality, but also some of the hurdles our specialty faces as we strive toward greater participation in the CMS quality incentive programs. Richard M. Rosenfeld, MD, MPH, several other volunteers associated with research and quality improvement, and I have joined our socioeconomic colleagues on the Ad Hoc Payment Workgroup (led by James C. Denneny III, MD) to discuss ways we can use our combined knowledge of research, quality, payment, and policy to address future models of payment being discussed at the highest levels of government and by the private payer community. The group just reviewed all of the different Academy guidance products as one of the first steps in this process and shared this information at the Board meeting in May. In terms of research, this year’s Centralized Otolaryngology Research Efforts (CORE) grants program was again a great success. For our members who are not as familiar with this program, the Academy deploys an extensive administrative effort with CORE. This allows for a streamlined, efficient process from advertising the program to our members, to providing a software solution for enhancing the grant review process, to assisting grantees with uploading applications, and in identifying reviewers for each specialty area. The Academy organizes and funds an intense, but gratifying two-day, one-night NIH-modeled study section meeting in Dallas during a weekend every March. This year a total of 41 grants were awarded $848,730 in funds dispersed through the academy and our sister societies. CORE Grantees will be recognized at the AAO-HNSF Annual Research Awards Ceremony, which will take place 10:30 am–11:50 am on Tuesday, October 1 at the AAO-HNSF Annual Meeting & OTO EXPOSM in Vancouver, Canada. Beginning as an idea discussed among a visionary group of thought leaders at a quality conference in 2006, our clinical practice guidelines development process has evolved to become “best in practice” as identified by the Agency for Healthcare Research and Quality and the Institute of Medicine. The Guidelines Task Force (led by Seth R. Schwartz, MD, MPH) has become another model of what can be accomplished when the Academy and specialty societies work together for the betterment of the specialty. As this article goes to press, we have 10 guideline products published and several more in press. These clinical knowledge products have become increasingly important as we broaden our efforts of demonstrating our commitment to quality in the practice of otolaryngology-head and neck surgery, including building our expertise in quality measure development and exploring registry methods that could assist the specialty in several ways. These could include helping members to participate in quality incentive programs set by the government and private payers. I hope you have time to read in detail about all of the projects that are underway as outlined in this month’s Bulletin. The expertise and passion of our committee and taskforce volunteer members certainly have helped us complete some exciting projects as we work to meet and exceed our strategic priorities for research and quality. Finally, I wish to shine light on our extraordinary academy staff members, led by Jean Brereton, MBA, who have been instrumental in the vision and operationalization of these important projects for our specialty. Please join me in thanking them and our volunteer Academy members for their tireless efforts and unparalleled commitment.
As you can tell from reading through these pages, there is an abundance of work being done on behalf of our members in the areas of quality and research. It is imperative, given all of the focus from external organizations including the government, private payers, and certifying boards (ABOto and the umbrella ABMS organization), that we are involved in initiatives that demonstrate the specialty’s continued focus on improving performance in practice. I want to highlight several of these initiatives in this column.
The Patient Safety and Quality Improvement (PSQI) Committee (led by David W. Roberson, MD, and Rahul K. Shah, MD) agreed to spearhead the AAO-HNS Foundation’s participation with the American Board of Internal Medicine (ABIM) Foundation’s Choosing Wisely® campaign last year. The main focus of the campaign was to identify tests and/or procedures that should be questioned and to engage patients in discussions about appropriateness of care. This was a great example of the specialties working together toward a common goal. The PSQI engaged other academy and foundation committees, the Specialty Society Advisory Council (SSAC) and the Guideline Task Force (GTF), in the development of an AAO-HNS list, and many of the suggestions came from AAO-HNS clinical practice guideline action statements. (Further information on Choosing Wisely appears in the PSQI summary on p. 32.) As the first surgical specialty to join the campaign, we were invited to participate in the Washington press conference in February at the Kaiser Family Foundation headquarters to roll out our list with other specialty societies. A commentary article on the campaign appeared in the April 2013 edition of Otolaryngology–Head and Neck Surgery.
The PSQI committee was also instrumental in working with the FDA on our members’ behalf when the FDA contacted us about its plans to issue a directive regarding the use of codeine post tonsillectomy and/or adenoidectomy. The PSQI Committee proactively emailed members once it was informed that the FDA was moving in this direction. The committee then kept in touch with the FDA as it came out with an alert in August warning of the risk of possible fatality when codeine is used in this clinical setting and when it ultimately issued a black box warning and contraindication in February. The FDA agreed to co-author a commentary with PSQI Committee co-chair, Dr. Roberson, and our Executive Vice President and CEO, David R. Nielsen, MD, which was published in the New England Journal of Medicine (http://bit.ly/NEJMdrug).
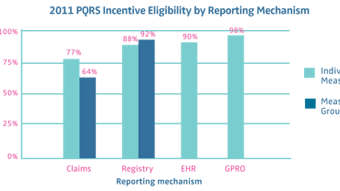
This year, Research and Quality Improvement has partnered even more effectively with the Physician Payment Policy (3P) Workgroup to address many of the tenets of healthcare reform relating to quality, including public reporting of physician data initiatives, Medicare incentive programs, and alternative payment models. In April, physician leaders from the 3P Workgroup and the PSQI Committee, along with staff from both business units, met with officials at the Centers for Medicare & Medicaid Services (CMS) to receive feedback and discuss not only the Academy’s significant accomplishments in the area of quality, but also some of the hurdles our specialty faces as we strive toward greater participation in the CMS quality incentive programs. Richard M. Rosenfeld, MD, MPH, several other volunteers associated with research and quality improvement, and I have joined our socioeconomic colleagues on the Ad Hoc Payment Workgroup (led by James C. Denneny III, MD) to discuss ways we can use our combined knowledge of research, quality, payment, and policy to address future models of payment being discussed at the highest levels of government and by the private payer community. The group just reviewed all of the different Academy guidance products as one of the first steps in this process and shared this information at the Board meeting in May.
In terms of research, this year’s Centralized Otolaryngology Research Efforts (CORE) grants program was again a great success. For our members who are not as familiar with this program, the Academy deploys an extensive administrative effort with CORE. This allows for a streamlined, efficient process from advertising the program to our members, to providing a software solution for enhancing the grant review process, to assisting grantees with uploading applications, and in identifying reviewers for each specialty area. The Academy organizes and funds an intense, but gratifying two-day, one-night NIH-modeled study section meeting in Dallas during a weekend every March. This year a total of 41 grants were awarded $848,730 in funds dispersed through the academy and our sister societies. CORE Grantees will be recognized at the AAO-HNSF Annual Research Awards Ceremony, which will take place 10:30 am–11:50 am on Tuesday, October 1 at the AAO-HNSF Annual Meeting & OTO EXPOSM in Vancouver, Canada.
Beginning as an idea discussed among a visionary group of thought leaders at a quality conference in 2006, our clinical practice guidelines development process has evolved to become “best in practice” as identified by the Agency for Healthcare Research and Quality and the Institute of Medicine. The Guidelines Task Force (led by Seth R. Schwartz, MD, MPH) has become another model of what can be accomplished when the Academy and specialty societies work together for the betterment of the specialty. As this article goes to press, we have 10 guideline products published and several more in press. These clinical knowledge products have become increasingly important as we broaden our efforts of demonstrating our commitment to quality in the practice of otolaryngology-head and neck surgery, including building our expertise in quality measure development and exploring registry methods that could assist the specialty in several ways. These could include helping members to participate in quality incentive programs set by the government and private payers.
I hope you have time to read in detail about all of the projects that are underway as outlined in this month’s Bulletin. The expertise and passion of our committee and taskforce volunteer members certainly have helped us complete some exciting projects as we work to meet and exceed our strategic priorities for research and quality. Finally, I wish to shine light on our extraordinary academy staff members, led by Jean Brereton, MBA, who have been instrumental in the vision and operationalization of these important projects for our specialty. Please join me in thanking them and our volunteer Academy members for their tireless efforts and unparalleled commitment.