Biologics for Chronic Rhinosinusitis with Nasal Polyps
A practical update on why, who, when, and which one.
Pedro Lança Gomes, MD, Amar Miglani, MD, Michael J. Marino, MD, and Devyani Lal, MD
The authors have no relevant conflicts of interest or financial disclosures.
Chronic rhinosinusitis (CRS) is a commonly prevalent condition in the United States afflicting 10.9%-13.4% of the adult population.1 Chronic rhinosinusitis with nasal polyps (CRSwNP) is estimated to affect 1.1% of the adult population in the U.S.1 CRSwNP detrimentally impacts quality of life, affecting the ability to breathe nasally, smell, and function normally.2,3 In addition, CRS can be associated with sleep impairment, anxiety, and depression.4 CRSwNP is generally considered to be driven by a predominant type 2 pattern of inflammation in the United States and the Western world;5 however, it is possible that more than one type of inflammation (e.g., type 1, type 2, and type 3) may coexist in the individual patient.5,6
The contemporary standard of care in treating CRSwNP includes therapeutics modulating or decreasing the inflammatory response in the sinonasal mucosa, with endoscopic sinus surgery (ESS) recommended for patients refractory to optimal medical therapy.2 Optimal medical therapy for CRSwNP as recommended by consensus guidelines includes high-volume saline rinse and topical nasal steroids with or without short courses of systemic corticosteroids.2,3 Contemporary approaches to surgery and postoperative maintenance medical therapy can be effective in the vast majority of CRSwNP patients.7,8 However, some CRSwNP patients can have particularly recalcitrant diseases unresponsive to standard of care therapy.8
Biologics are monoclonal antibodies developed through recombinant technology. They act by targeting a specific protein considered germane in the pathogenesis of a disease.9 Three biologics targeting type 2 inflammation in CRSwNP have recently been approved by the U.S. Food and Drugs Administration (FDA): dupilumab, mepolizumab, and omalizumab.
In this article, we will discuss the treatment of CRSwNP with biologics, with a practical focus on rationale (why), indications (when, whom), and selection of biologic agents (which one).
Why: The Evidence for Biologics in CRSwNP
Three biological options are approved by the FDA for the treatment of nasal polyps target type 2 inflammation via different approaches. Omalizumab is a monoclonal antibody to immunoglobulin E (IgE); dupilumab works against the alpha subunit receptor for interleukin-4 (IL-4), consequently causing blockade of IL-4 and IL-13; and mepolizumab is an anti-interleukin-5 monoclonal antibody. The product label for biologics states these to be add-on maintenance treatments for adult patients who have inadequately controlled nasal polyps. Other examples of biologic agents being tested for CRSwNP are reslizumab (anti IL-5), benralizumab (anti IL-5Rα), etokimab (anti IL-33), and tezepelumab (anti-TSLP), but the data from these trials have not been published.
Table 1 summarizes the mechanism of action for each of the approved biologics, while Figure 1 also depicts where these biologics target nasal polyp pathogenesis. Table 2 summarizes the phase 3 randomized clinical trials (RCT) conducted for each approved biologic agent.
Table 1. Mechanism of action of the three available biologic agents for CRSwNP.
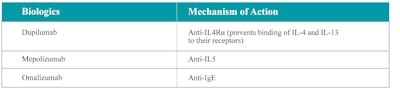
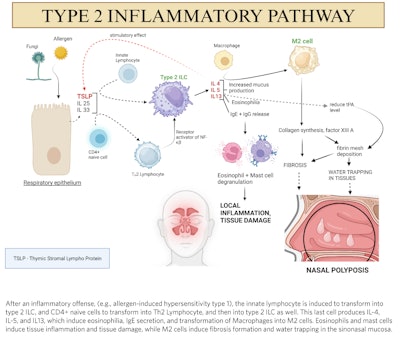 Figure 1. Pathogenesis of nasal polyps. (Figure created by Nitish Kumar, MBBS, MS, using biorender.com.)
Figure 1. Pathogenesis of nasal polyps. (Figure created by Nitish Kumar, MBBS, MS, using biorender.com.)
Table 2. Phase 3 RCT designs on biologics for CRSwNP.
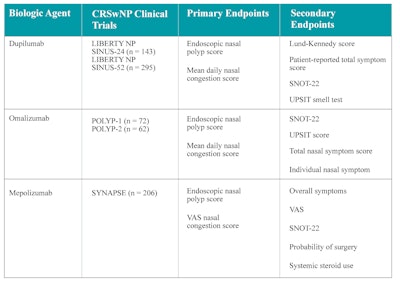 VAS = visual analogic scale; SNOT-22 = 22-item Sino-Nasal Outcome Test; UPSIT = University of Pennsylvania Smell Identification Test.
VAS = visual analogic scale; SNOT-22 = 22-item Sino-Nasal Outcome Test; UPSIT = University of Pennsylvania Smell Identification Test.
Omalizumab was initially approved by the FDA for the treatment of chronic idiopathic urticaria and moderate to severe asthma. Its use for nasal polyps was theorized based on the high nasal tissue expression of IgE in this condition. Two randomized phase 3 trials (POLYP 1 and POLYP 2) were conducted for use in nasal polyps and concluded in 2020.10 Dupilumab was first approved for noncontrolled moderate to severe atopic dermatitis. Two randomized phase 3 trials on use in nasal polyps (LIBERTY NP SINUS 24 and LIBERTY NP SINUS 52) were concluded in 2019.11 Mepolizumab was first approved for severe eosinophilic asthma and is also approved for patients with eosinophilic granulomatosis with polyangiitis (EGPA). A randomized phase 3 trial (SYNAPSE 52) for nasal polyps was concluded in 2021.12 Table 2 details the outcomes of primary and secondary endpoints for each RCT on biologic use in nasal polyps. In general, dupilumab, mepolizumab, and omalizumab were all successful in demonstrating increased efficacy in the primary end point of decreasing the nasal polyp size.
Who: CRSwP Patients Who Are Candidates for Biologic Therapy
Table 3 shows the primary end point results of RCTs conducted for the biologics approved for nasal polyps. In all the RCTs, subjects included had nasal polyp score of ≥5 (out of a total score of 8; 4 on each side). In all trials, intranasal corticosteroid sprays (mometasone furoate 200-400 mcg/day, or equivalent topical corticosteroid) were included as standard of care in both study and control arms. A majority of subjects had undergone previous ESS in each trial.10,11,12
Table 3. Data for the primary endpoints on each RCT phase 3 trial.
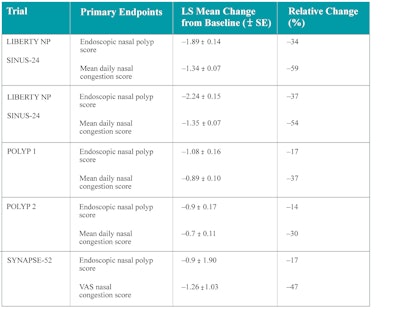 SE = standard error; VAS = visual analogic scale; LS = least square.
SE = standard error; VAS = visual analogic scale; LS = least square.
Source: Miglani A, et al. A comparative analysis of endoscopic sinus surgery versus biologics for treatment of chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2023;13(2):116-128. doi:10.1002/alr.23059
Currently, optimal indications for the use of biologics may be best used over standard of care are yet to be defined. Although some patients may be super responders, responses in other patients may be underwhelming. Indeed, biologics appear to work effectively in approximately 60%-70% of patients they are used in, leading to questions regarding which patients may be best suited to benefit.13
The RCTs of dupilumab and mepolizumab extended up to 52 weeks,11,12 while the RCTs for omalizumab extended up to 24 weeks.10 Questions remain unanswered regarding the optimal length of treatment for nasal polyps with biologics. It is not clearly understood if nasal polyps will recur after cessation of biologic treatment or if patients will need lifelong treatment with these drugs. It is not known how long patients need to be on treatment until a conclusion of “nonresponse” can be made. Also, protocols to safely de-escalate therapy with biologics are unclear in patients with good long-term responses.
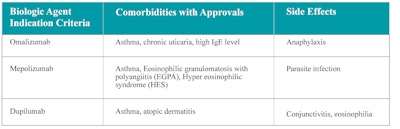
Table 4 outlines the most common side effects associated with each of the approved biologics for nasal polyps. In general, the use of these biologics appears to be associated with a low rate of adverse effects, but transient hypereosinophilia with duplilumab is a significant concern in precipitating EGPA (or unmasking it).
Table 4. Factors to consider when choosing a biologic agent.
When?
There are no universal guidelines as to when it is most appropriate to use biologics in the treatment of nasal polyps. The most current guidelines published in the International Consensus Statement on Allergy and Rhinosinusitis (ICARS 2021)2 did not address biological therapy in the algorithm proposed for the treatment of nasal polyps. In the absence of this, Han et. al. proposed an algorithm starting with the use of topical nasal steroids and consideration of a short burst of oral corticosteroids as a first-line approach.14 For recalcitrance, second-line therapy by delivery of topical nasal steroids through nonconventional methods such as an exhalation device–delivered fluticasone and the use of high-volume irrigations impregnated with topical steroids was proposed, prior to advancing to surgery; biologics were proposed for recalcitrance after surgery. Primary deployment of biologics was reserved for those judged unsuitable for ESS who are choosing not to undergo surgery.14
The 2020 European position paper on sinusitis (EPOS) proposed the use of biologics may be appropriate in patients with bilateral nasal polyposis who had undergone previous endoscopic sinus surgery and met three of five additional criteria: evidence of type 2 inflammation, use of systemic steroid treatment chronically or over twice a year, SNOT-22 score ≥ 40, reduced smell, and asthma requiring inhaled steroids.3 A consensus statement from a forum of otolaryngologists and allergy immunologists, EUFOREA, suggested the use of biologics in patients with bilateral nasal polyps who had undergone sinus surgery in the past and met three of the five additional criteria: evidence of type 2 inflammation, need for systemic corticosteroid (≥ 2 courses in the past year), significantly impaired quality of life, significant loss of smell, diagnosis of comorbid asthma. For patients with no history of sinus surgery, four of the above criteria would be needed to be eligible for biological treatment.15,16
Multispecialty comprehensive management at the outset is optimal for managing CRSwNP, especially when asthma is coexistent. Shared decision-making in CRSwNP must always be a prioritized approach, considering personalized factors relevant to the disease state and the patient’s values and preferences.17 Figure 2 depicts the authors’ algorithm based on a review of trial data and guidelines from Han et al.,14 EPOS,3 and EUPHORIA15 criteria. We propose the use of topical steroid sprays with or without a short (< 30 days) burst of oral corticosteroid as the first-line approach. After one month, if symptoms and objective signs of disease persist, the use of exhalation (breath-powered) delivery systems with fluticasone (EDS-FLU) or topical corticosteroids delivered via high-volume nasal irrigation may be discussed as an alternative. For recalcitrant symptoms and disease,14 ESS is proposed for patients without contraindications to general anesthesia.
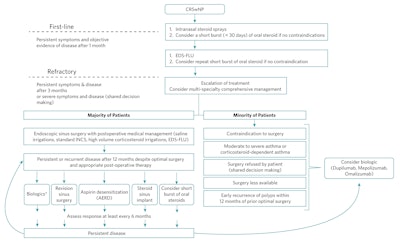 Figure 2. Modified algorithm.
Figure 2. Modified algorithm.
EDS-FLU = exhalation breath-powered corticosteroid devices; AERD = aspirin-exacerbated respiratory disease; INCS = intranasal corticosteroid
*May be also an option in patients with a history of polyp recurrence within 12 months of a previous surgery.
On the other hand, therapy with biologics may be prioritized over surgery in the following scenarios: if there is a contraindication to surgical/anesthetic procedures or the patient declined surgery, has a history of early recurrence of polyps within 12 months of prior optimal surgery, or has moderate-to-severe corticosteroid-dependent asthma.
Maintenance therapy after ESS for CRSwNP includes saline irrigation and topical intranasal steroid therapy. If persistent or recurrent disease is noted despite appropriate surgery and postoperative care, biologic therapy may be indicated. Biologics immediately after surgery may be an option in patients with a history of polyp recurrence within 12 months of previous surgery.15 Other options for recurrence include revision surgery with secondary procedures such as a modified Lothrop approach, aspirin desensitization for aspirin-exacerbated respiratory disease (AERD), corticosteroid sinus implant, and short bursts of oral corticosteroids.
How Long?
If a biological agent is started, the patient should be evaluated at least every six months to assess the treatment’s efficacy.18 Figure 3 illustrates a modification of the algorithm for follow-up of patients on biologic treatment proposed by EUFORIA.15
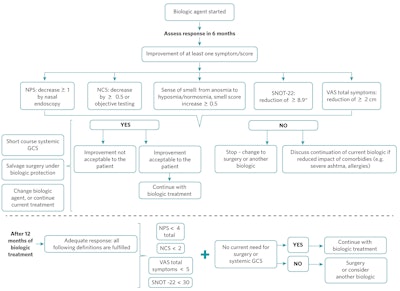 Figure 3. Biologic therapy follow-up modified algorithm.
Figure 3. Biologic therapy follow-up modified algorithm.
NPS = nasal polyp score; NCS = nasal congestion score; SNOT-22 = 22-item Sino-Nasal Outcome Test; VAS = visual analogic scale; GCS = glucocorticosteroid
*Minimal clinically important difference
After six months of biologic treatment, if there is improvement of at least one symptom/score (decrease ≥1 in nasal polyp score; decrease ≥0.5 on nasal congestion score or objective testing; from anosmia to hyposmia/normosmia, smell score increase >0.5; SNOT-22 reduction ≥8.9; or reduction ≥2 cm on total symptoms visual analogic scale) and this improvement was considered satisfactory by the patient, treatment with biologic should continue for at least six more months.
If no sinonasal symptom improvement was noticed, the biologic should be stopped, unless it was associated with a reduced impact of comorbidities (e.g., severe asthma, allergies). Surgical intervention and/or another biologic agent could be offered to the patient.
If there was an improvement that was not considered acceptable to the patient, options would be salvage surgery under biologic protection, an additional short course of systemic glucocorticosteroid, another biologic agent, or continuing with biologic treatment for a new assessment in 12 months.
After 12 months of biologic treatment, if the patient presented with adequate response (nose polyp score <4, nose congestion score <2, VAS total symptoms <5, and SNOT-22 <30), and did not require surgery or systemic glucocorticosteroid courses, treatment with the current biologic may continue. On the other hand, if the response was inadequate, or the patient required surgery or systemic glucocorticosteroid courses, additional surgery and/or another biologic agent can be considered.
Which One
Currently, there are no head-to-head trials comparing the efficacy of dupilumab, mepolizumab, and omalizumab. Therefore, the data on which biologic may be best suited for use in the individual patient is not clear. Secondary analysis of the data published from the RCTs showed dupilumab to be the most efficacious in primary and secondary endpoints, including in smell restoration.19,20
Rank, et al. published in 2023 an evidence-based guideline for the medical management of chronic rhinosinusitis with nasal polyposis.19 The “GRADE” criteria (Grading of Recommendations, Assessment, Development, and Evaluations) is a framework summary of evidence for a systematic approach to making clinical practice recommendations.21 The utilization of GRADE criteria meant that the authors excluded ESS from the management algorithm. In addition, the algorithm did not consider novel methods of intranasal corticosteroid therapy delivery such as high-volume nasal irrigations and exhalation device systems.
The authors compared various quality of life metrics (SNOT-22 and nasal obstruction VAS), UPSIT score, need for rescue surgery, adverse events, and surrogate outcomes (polyp size, Lund-Kennedy score, Lund-Mackay score). Table 5 displays the comparisons made between dupilumab, omalizumab, and mepolizumab.19 Dupilumab was superior to omalizumab and mepolizumab in improvement of quality of life as measured by SNOT-22 score, nasal obstruction score, smell improvement, decrease in need for rescue oral corticosteroids, and decrease in need for ESS. Dupilumab was also superior for improvement in nasal polyp size, endoscopic appearance via (Lund-Kennedy endoscopy score), and CT scores (Lund-Mackay CT score).
Table 5. Comparison between approved biologics for CRSwNP expressed in mean difference (MD) and 95% CI.
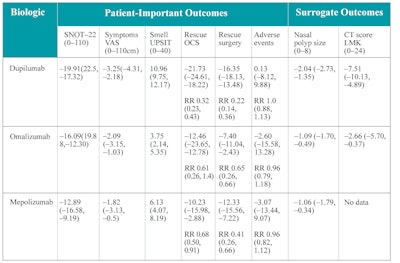 VAS = visual analogic scale; SNOT-22 = 22-item Sino-Nasal Outcome Test; UPSIT = University of Pennsylvania Smell Identification Test; CT = computed tomography; LMK = Lund-McKay; RR = relative risk; OCS = oral corticosteroid
VAS = visual analogic scale; SNOT-22 = 22-item Sino-Nasal Outcome Test; UPSIT = University of Pennsylvania Smell Identification Test; CT = computed tomography; LMK = Lund-McKay; RR = relative risk; OCS = oral corticosteroid
Source: Rank MA, et al. The Joint Task Force on Practice Parameters GRADE guidelines for the medical management of chronic rhinosinusitis with nasal polyposis. J Allergy Clin Immunol. 2023;151(2):386-398. doi:10.1016/j.jaci.2022.10.026
Unfortunately, since ESS, which is the standard of care, was not considered in this algorithm, the recommendation to consider biologic therapy if the patient has failed to improve with intranasal steroid sprays after four weeks was made.19 Although several caveats were proposed in this recommendation, these do not feature prominently in the decision-making algorithm. The lack of incorporation of surgery in this algorithm impacts the real-world utilization of biologics, especially by allergists, who form the primary readership of the article’s journal.19
Table 4 also summarizes conditions other than nasal polyps with FDA approvals for these biologics. These comorbidities may be helpful when deciding on the choice of the biologic agent. For example, if the patient has moderate-to-severe serum eosinophilia, dupilumab might not be used since it can increase peripheral eosinophilia and may unmask or precipitate EGPA. Instead, mepolizumab may be a better choice. Additionally, mepolizumab is also FDA-approved for EGPA.22 Interdisciplinary dialogue guides both health practitioners and patients toward the best medical decision.17
Cost of Biologics vs. Standard of Care
Treatment with biologics is expensive for patients and health systems. A cost-effectiveness analysis published in 2019 assessed all biologic therapies approved for asthma. The annual expense estimation for biologic wholesale acquisition was between US$30,000 and US$40,000. The costs of biologics would need to be reduced between 62% and 80% from the wholesale acquisition cost to meet cost-effectiveness thresholds.23
In 2014 the overall cost for outpatient ESS in the U.S. ranged between $8,200 and $10,500 per case.24 A cost-utility analysis using a Markov model showed that the ESS strategy, including primary and revision surgery, was more cost-effective than dupilumab for CRSwNP.25 Based on this analysis, the ESS strategy cost was $50,436.99 and produced 9.80 QALYs. On the other hand, the dupilumab treatment strategy cost was $536,420.22 and produced 8.95 QALYs. In addition, a cost-utility analysis comparing ESS to dupilumab revealed that, at any yearly cost of dupilumab greater than $855, surgery was more cost-effective.25
Given that there is no current high-quality data on the length of treatment needed with biologics for nasal polyps and that standard of care incorporating ESS is beneficial in most patients for long-term symptom improvement and control of disease, the added cost to healthcare secondary to indiscriminate biologic use must be assessed.19,25
Even though there is no direct comparison between biologics and surgery, Miglani et al. published in 2023 an indirect comparison using subjects similar to subjects in the dupilumab trials. Their study revealed that at 24 weeks ESS was significantly better in terms of SNOT-22 improvement than dupilumab and omalizumab. ESS was also superior in terms of reduction of nasal polyp score and was comparable in terms of smell measurement. At 52 weeks ESS was comparable to dupilumab in terms of SNOT-22 improvement and remained superior in terms of nasal polyp score reduction.26 Table 6 summarizes their findings on comparing dupilumab with ESS at 24 and 52 weeks.
Table 6. Comparison between dupilumab and ESS for the treatment of nasal polyps.
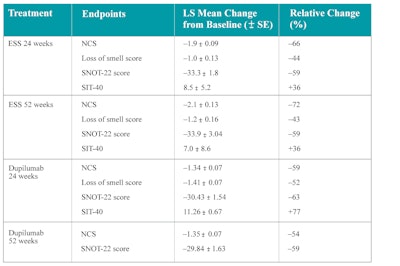 SNOT-22 = 22-item Sino-Nasal Outcome Test; LS = least square; NCS = nasal congestion score; SIT-40 = smell identification test-40; SE = standard error.
SNOT-22 = 22-item Sino-Nasal Outcome Test; LS = least square; NCS = nasal congestion score; SIT-40 = smell identification test-40; SE = standard error.
Source: Miglani A, et al. A comparative analysis of endoscopic sinus surgery versus biologics for treatment of chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2023;13(2):116-128. doi:10.1002/alr.23059
Summary
Three biologics targeting type 2 inflammation have recently been approved by the FDA for nasal polyps: dupilumab, mepolizumab, and omalizumab. These appear to reduce nasal polyp size and positively impact sinonasal symptoms and quality of life with varying efficacy. Many questions on the use of biologics for nasal polyps currently remain unanswered regarding the length of treatment, who benefits most, and whether biologics should be used with, without, before, or after surgery. Post-marketing surveillance and trials addressing those specific questions are needed to answer these questions.
The role of biologics for patients diagnosed with allergic fungal rhinosinusitis and mild sinonasal disease is still not clear since those conditions were excluded from the trials on omalizumab, dupilumab, and mepolizumab. In summary, a thoughtful approach considering multiple patient, disease, and drug factors should be used when choosing biologic as a therapeutic option. Shared decision-making can be a valuable approach for both patients and healthcare providers, given that there is no universally accepted consensus guideline.17
References
- Palmer JN, Messina JC, Biletch R, Grosel K, Mahmoud RA. A cross-sectional, population-based survey of U.S. adults with symptoms of chronic rhinosinusitis. Allergy Asthma Proc. 2019;40(1):48-56. doi:10.2500/aap.2019.40.4182
- Orlandi RR, Kingdom TT, Smith TL, Bleier B, DeConde A, Luong AU, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021;11(3):213-739. doi:10.1002/alr.22741
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Vol 103.
- Mullol J, Azar A, Buchheit KM, Hopkins C, Bernstein JA. Chronic rhinosinusitis with nasal polyps: quality of life in the biologics era. J Allergy Clin Immunol Pract. 2022;10(6):1434-1453.e9. doi:10.1016/j.jaip.2022.03.002
- Kato A, Schleimer RP, Bleier BS. Mechanisms and pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol. 2022;149(5):1491-1503. doi:10.1016/j.jaci.2022.02.016
- Stevens WW, Schleimer RP, Kern RC. Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract. 2016;4(4):565-572. doi:10.1016/j.jaip.2016.04.012
- Loftus CA, Soler ZM, Desiato VM, Koochakzadeh S, Yoo F, Storck KA, et al. Factors impacting revision surgery in patients with chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2020;10(3):289-302. doi:10.1002/alr.22505
- DeConde AS, Mace JC, Levy JM, Rudmik L, Alt JA, Smith TL. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope. 2017;127(3):550-555. doi:10.1002/lary.26391
- Malik B, Ghatol A. Understanding how monoclonal antibodies work. In: StatPearls [Internet]. Updated June 26, 2023. https://www.ncbi.nlm.nih.gov/books/NBK572118/
- Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020;146(3):595-605. doi:10.1016/j.jaci.2020.05.032
- Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638-1650. doi:10.1016/S0140-6736(19)31881-1
- Han JK, Bachert C, Fokkens W, Desrosiers M, Wagenmann M, Lee SE, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9(10):1141-1153. doi:10.1016/S2213-2600(21)00097-7
- Carney AS, Smith PK. Current understanding of the role of eosinophils in CRSwNP and implications for treatment with mepolizumab and benralizumab. Am J Rhinol Allergy. 2023;37(2):175-181. doi:10.1177/19458924221149270
- Han JK, Bosso JV, Cho SH, Franzese C, Lam K, Lane AP, et al. Multidisciplinary consensus on a stepwise treatment algorithm for management of chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2021;11(10):1407-1416. doi:10.1002/alr.22851
- Bachert C, Han JK, Wagenmann M, Hosemann W, Lee SE, Backer V, et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: definitions and management. J Allergy Clin Immunol. 2021;147(1):29-36. doi:10.1016/j.jaci.2020.11.013
- Fokkens WJ, Lund V, Bachert C, Mullol J, Bjermer L, Bousquet J, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy. 2019;74(12):2312-2319. doi:10.1111/all.13875
- Ramkumar SP, Lal D, Miglani A. Considerations for shared decision-making in treatment of chronic rhinosinusitis with nasal polyps. Front Allergy. 2023;4. doi:10.3389/falgy.2023.1137907
- Bachert C, Desrosiers MY, Hellings PW, Laidlaw TM. The Role of Biologics in Chronic Rhinosinusitis with Nasal Polyps. J Allergy Clini Immunol Pract. 2021;9(3):1099-1106. doi:10.1016/j.jaip.2020.11.017
- Rank MA, Chu DK, Bognanni A, Oykhman P, Bernstein JA, Ellis AK, et al. The Joint Task Force on Practice Parameters GRADE guidelines for the medical management of chronic rhinosinusitis with nasal polyposis. J Allergy Clin Immunol. 2023;151(2):386-398. doi:10.1016/j.jaci.2022.10.026
- Oykhman P, Paramo FA, Bousquet J, Kennedy DW, Brignardello-Petersen R, Chu DK. Comparative efficacy and safety of monoclonal antibodies and aspirin desensitization for chronic rhinosinusitis with nasal polyposis: a systematic review and network meta-analysis. J Allergy Clin Immunol. 2022;149(4):1286-1295. doi:10.1016/j.jaci.2021.09.009
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation — determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013;66(7):726-735. doi:10.1016/j.jclinepi.2013.02.003
- Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921-1932. doi:10.1056/nejmoa1702079
- Anderson WC, Szefler SJ. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol. 2019;122(4):367-372. doi:10.1016/j.anai.2019.01.018
- Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope. 2015;125(7):1547-1556. doi:10.1002/lary.25180
- Scangas GA, Wu AW, Ting JY, Metson R, Walgama E, Shrime MG, et al. Cost utility analysis of dupilumab versus endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps. Laryngoscope. 2021;131(1):E26-E33. doi:10.1002/lary.28648
- Miglani A, Soler ZM, Smith TL, Mace JC, Schlosser RJ. A comparative analysis of endoscopic sinus surgery versus biologics for treatment of chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2023;13(2):116-128. doi:10.1002/alr.23059