Research and Quality: Creating the tools you need
The AAO-HNSF Research and Quality Business Unit comprises the following key Foundation initiatives: the Reg-ent℠ Clinical Data Registry; Quality Measures Development; the Guidelines Task Force (Clinical Practices Guideline and Clinical Consensus Statement development, implementation, and dissemination); and the Centralized Otolaryngology Research Efforts (CORE) grants program.

The Research and Quality Business Unit staff, in conjunction with our dedicated member volunteers, has been diligently focused on developing products and tools to help members navigate the ever-changing landscape of research, quality, and performance measurement. It is our goal to have the tools and information and processes in place that our members need to successfully practice and participate with these endeavors.
Reg-ent℠ registry
To that end, we have, in the past year, signed up more than 1,800 participants in the first otolaryngology-specific national clinical data registry, Reg-ent. We are approaching the conclusion of the first year of participation for the majority of members who joined the registry in the third quarter of 2016. A subset of those members reported through Reg-ent to CMS for the final year of the PQRS program and the expectation is that many more will be reporting to the Merit-based Incentive Payment System (MIPS) for 2017. Many of the practices representing these physicians are working closely with Reg-ent and our registry partner, FIGmd, to ensure data are captured and mapped appropriately from their EHR for submission to Reg-ent.
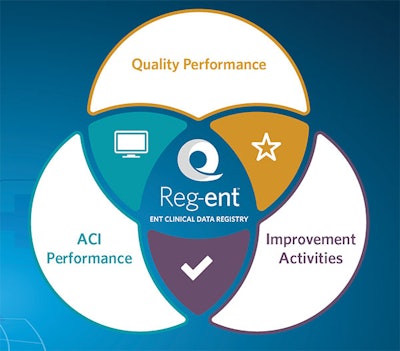 Reg-ent’s enhanced technology platform will accommodate all required 2017 MIPS reporting categories including Quality Performance, Advancing Care Information (ACI), and Improvement Activities (IA).
Reg-ent’s enhanced technology platform will accommodate all required 2017 MIPS reporting categories including Quality Performance, Advancing Care Information (ACI), and Improvement Activities (IA).Reg-ent has multiple benefits beyond serving as a reporting tool. As we grow the registry with increasing numbers of patients and patient visits, we are creating a national clinical data repository for our specialty. Through Reg-ent, we will be able to perform comparative effectiveness research using prospectively collected clinical data. Reg-ent will also provide a forum for pre- and post-market device monitoring, prepare and assist members with meeting requirements for government and private payer quality initiatives, and assist members with submitting required data to the American Board of Otolaryngology for Maintenance of Certification (MOC) Part IV. We look forward to having all otolaryngologist-head and neck surgeons participate in Reg-ent.
A key part of the registry development is creation of meaningful quality measures for Reg-ent. Last year, the Reg-ent Executive Committee convened seven Clinical Advisory Committees (CACs) to represent all of the subspecialties within otolaryngology. In the coming pages, you will see the work that has taken place in the last 12 months with the development of meaningful measures for our specialty. Many of the measures initiatives have benefited greatly from the fact that we have a robust clinical practice guideline process and have volunteers who have dedicated significant time and effort to the continued development of guidelines on topics across the specialty. This year, as in past years, we have developed several new guidelines and updated several existing guidelines. In addition, several new consensus statements are currently under development.
Outcomes Research and Evidence-Based Medicine

OREBM also continues to undertake systematic reviews and is currently evaluating vocal outcomes after cardiac surgery in pediatric patients and validated instruments for outcome measures. In addition, OREBM members had six Miniseminars accepted to the AAO-HNSF 2017 Annual Meeting & OTO Experience (Controversies in Parotid Surgery: Is There Evidence?; Evidence-Based Management of Ménière’s Disease; Kids Today: Rapid Review of Guidelines and Consensus; Recent Publications that Could Change Your Practice; Registries and Databases: How and Why?; and The Latest (and Greatest?) Devices in Ears, Nose, and Sleep). The committee also sponsors a recurring feature in the Bulletin, which highlights notable data in a spotlight feature. The group contributes to the “Evidence-Based Medicine in Otolaryngology” educational journal series with ongoing work. In addition, OREBM members have volunteered their time to prepare for the planned update of the tonsillectomy guideline. The committee has engaged in joint projects with other Academy groups, including the Medical Devices and Drugs Committee, the Rhinology and Paranasal Sinuses Committee, the Equilibrium Committee, the Guidelines Task Force, and Reg-ent. All in all, OREBM remains highly active in advancing the mission of the committee and our Academy.
Patient Safety Quality Improvement
The Patient Safety Quality Improvement Committee, under the leadership of Julie C. Goldman, MD, and Michael J. Brenner, MD, has been involved in several key Foundation initiatives, and committee members continue to participate at high levels with these projects. This past year, the committee developed and submitted five Miniseminar topics for the 2017 Annual Meeting in Chicago (Management of the Difficult Airway; Promoting a Culture of Constant Improvement at Your Institution; QI to Develop Your Career; Medical Instrumentation and Sterilization in the Office; and Management of Cognitive Bias). QI to Develop Your Career was accepted for presentation.
Also, 36 new applicants applied to be on the PSQI Committee, which speaks to the interest and commitment of our members to quality and patient safety within the specialty. The committee is represented on the panel to update the clinical practice guideline on tonsillectomy. In addition, four committee members reviewed and provided feedback on a perioperative antibiotic survey submitted by the department of otolaryngology at Vanderbilt University.
Members and staff continue to monitor the national quality landscape organizations and participate in the American College of Surgeons Surgical Quality Alliance (SQA). Dr. Brenner attended the National Patient Safety Summit last August and brought back valuable information to the committee. This year, the committee is working on a white paper covering the issue of in-office sterilization and is considering cosponsoring a guideline submission on VTE prophylaxis. Just recently, several PSQI members volunteered to participated in a PSQI Assessment for Residence project. The PSQI continues to contribute greatly to the advancement of quality and safety within the specialty.
This issue of the Bulletin outlines our 2017 Centralized Otolaryngology Research Efforts (CORE) awardees on page 24. Please take time to review the list of those receiving awards and the list of grants which were conferred. We congratulate all recipients.