Clinical Practice Guideline: Opioid Prescribing for Analgesia After Common Otolaryngology Operations
Opioid use disorder (OUD), which includes misuse, abuse, and overdose of opioids, is an epidemic in the United States.

Opioid use disorder (OUD), which includes misuse, abuse, and overdose of opioids, is an epidemic in the United States. According to data from the National Survey on Drug Use and Health, more than six million people ages 12 or older misuse prescription pain relievers every year in the United States.1 Additionally, studies have shown that there is a significant risk of chronic opioid use even when used as short-term treatment for pain.2
“As otolaryngology-head and neck surgeons, we can help reduce the risk of OUD among our patients and their families. This CPG focuses on multimodal analgesia and judicious use of opioids for common otolaryngology procedures,” said Samantha Anne, MD, MS, Chair of the Guideline Development Group (GDG). James "Whit" Mims, MD, served as Assistant Chair, and David E. Tunkel, MD, and Richard M. Rosenfeld, MD, MPH, MBA, served as Methodologists.
The purpose of this specialty-specific guideline is to provide evidence-informed recommendations on postoperative management for pain in common otolaryngologic surgical procedures, with a focus on opioids.
In addition, it allows identification of quality improvement opportunities in postoperative pain management of common otolaryngologic surgical procedures. Employing the key action statements from this CPG can help to reduce the variation in care across the specialty and improve postoperative pain control while reducing the risk of OUD.
“Many times opioids are prescribed in large quantities for procedures that are associated with mild to moderate pain, such as parathyroidectomy, thyroidectomy, and otologic surgeries. The number of opioids prescribed for these procedures can be reduced, especially if appropriate multimodal analgesia is used,” says Dr. Anne. “The guideline also emphasizes the importance of counseling patients and identifying patient- and procedure-related factors that can inform shared decision-making.”
The guideline addresses assessment of patients for OUD risk factors, counseling on pain expectations, and identifying factors that can impact pain duration and/or severity. It also discusses the use of multimodal analgesia as first-line treatment and responsible use of opioids. Lastly, safe disposal of unused opioids is discussed.
The guideline reviews the healthcare burden caused by OUD. It highlights research on opioid prescribing and misuse in the U.S. as well as the mortality attributed to opioid overdoses. Additionally, it presents data on overprescribing of opioids for postoperative pain and the diversion of unused opioid medication.
Guideline Key Action Statements (KASs)
KAS 1: Expected Pain (recommendation)
Prior to surgery, clinicians should advise patients and others involved in the postoperative care about the expected duration and severity of pain.
KAS 2: Modifying Factors (recommendation)
Prior to surgery, clinicians should gather information specific to the patient that modifies severity and/or duration of pain.
KAS 3A: Risk Factors for Opioid Use Disorder (strong recommendation)
Prior to surgery, clinicians should identify risk factors for OUD when analgesia using opioids is anticipated.
KAS 3B: Patients at Risk for Opioid Use Disorder (recommendation)
In patients at risk for OUD, clinicians should evaluate the need to modify the analgesia plan.
KAS 4: Shared Decision Making (recommendation)
Clinicians should promote shared decision making by informing patients of the benefits and risks of postoperative pain treatments that include nonopioid analgesics, opioid analgesics, and nonpharmacologic interventions.
KAS 5: Multimodal Therapy (recommendation)
Clinicians should develop a multimodal treatment plan for managing postoperative pain.
KAS 6: Nonopioid Analgesia (strong recommendation)
Clinicians should advocate for nonopioid medications as first-line management of pain after otolaryngologic surgery.
KAS 7: Opioid Prescribing (recommendation)
When treating postoperative pain with opioids, clinicians should limit therapy to the lowest effective dose and the shortest duration.
KAS 8A: Patient Feedback (recommendation)
Clinicians should instruct patients and caregivers how to communicate if pain is not controlled or if medication side effects occur.
KAS 8B: Stopping Pain Medications (recommendation)
Clinicians should educate patients to stop opioids when pain is controlled with nonopioids and stop all analgesics when pain has resolved.
KAS 9: Storage and Disposal of Opioids (strong recommendation)
Clinicians should recommend that patients (or their caregivers) store prescribed opioids securely and dispose of unused opioids through take-back programs or another accepted method.
KAS 10: Assessment of Pain Control with Opioids (recommendation)
Clinicians should inquire, within 30 days of surgery, whether the patient has stopped using opioids, has disposed of unused opioids, and was satisfied with the pain management plan.
The GDG included 16 members representing otolaryngology-head and neck surgery generalists and subspecialists, pain management, nursing, and consumers. The CPG is intended for otolaryngologists who perform surgery and clinicians who manage pain after surgical procedures. The target patients for the guideline are any patients treated for anticipated or reported pain within the first 30 days after undergoing common otolaryngologic procedures.
The Opioid Prescribing for Analgesia After Common Otolaryngology Operations CPG was created using the methods listed in the AAO-HNSF “Clinical Practice Guideline Development Manual, Third Edition.” (https://journals.sagepub.com/doi/full/10.1177/0194599812467004)
The full guideline and other resources are available at www.entnet.org/opioidscpg and in Otolaryngology–Head and Neck Surgery as published at www.otojournal.org.
Guideline authors:
Samantha Anne, MD, MS (Chair); James W. Mims, MD (Assistant Chair); David E. Tunkel, MD (Methodologist); Richard M. Rosenfeld, MD, MPH, MBA (Methodologist); David S. Boisoneau, MD; Michael J. Brenner, MD; John D. Cramer, MD; David Dickerson, MD; Sandra A. Finestone, PsyD; Adam J. Folbe, MD, MS; Deepa J. Galaiya, MD; Anna H. Messner, MD; Allison Paisley, CRNP; Ahmad R. Sedaghat, MD, PhD; Kerstin M. Stenson, MD; Angela K. Sturm, MD; Erin M. Lambie, MS, MPH; Nui Dhepyasuwan, MEd; and Taskin M. Monjur
Endorsed by:
American Academy of Facial Plastic and Reconstructive Surgery (AAFPRS), American Broncho-Esophagological Association (ABEA), American Head and Neck Society (AHNS), American Neurotology Society (ANS), American Otological Society (AOS), American Rhinologic Society (ARS), American Society of Pediatric Otolaryngology (ASPO), Society of Otorhinolaryngology and Head-Neck Nurses (SOHN), The Triological Society
Disclaimer:
This guideline is not intended as the sole source of guidance in prescribing opioids and/or analgesics for common otolaryngologic procedures. Rather, it is designed to assist clinicians by providing an evidence-informed framework for decision-making strategies. The guideline is not intended to replace clinical judgment or establish a protocol for management for all individuals with pain after otolaryngologic surgery and may not provide the only appropriate approach to managing postoperative pain. As medical knowledge expands, and technology advances, clinical indicators and guidelines are promoted as conditional and provisional proposals of what is recommended under specific conditions but are not absolute. Guidelines are not mandates. They do not and should not purport to be a legal standard of care. The responsible physician, in light of all circumstances presented by the individual patient, must determine the appropriate treatment. Adherence to these guidelines will not ensure successful patient outcomes in every situation. The AAO-HNSF emphasizes that these clinical guidelines should not be deemed to include all proper treatment decisions or methods of care, or to exclude other treatment decisions or methods of care reasonably directed to obtaining the same results.
References:
1. Center for Behavioral Health Statistics and Quality. Results from the 2014 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration (SAMHSA), U.S. Department of Health and Human Services. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2014/NSDUH-DetTabs2014.pdf. Published 2014. Accessed December 8, 2019.
2. Lawal OD, Gold J, Murthy A, et al. Rate and Risk Factors Associated With Prolonged Opioid Use After Surgery: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3(6):e207367.
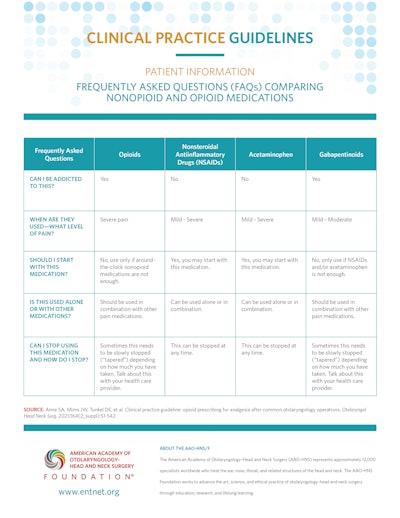
Patient Information: Frequently Asked Questions (FAQs) Comparing Nonopioid and Opioid Medications
Click Here to Download PDF
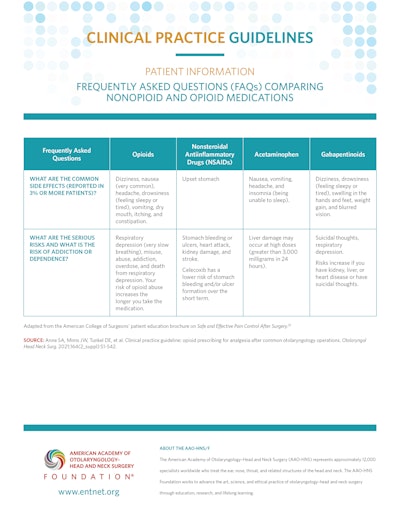
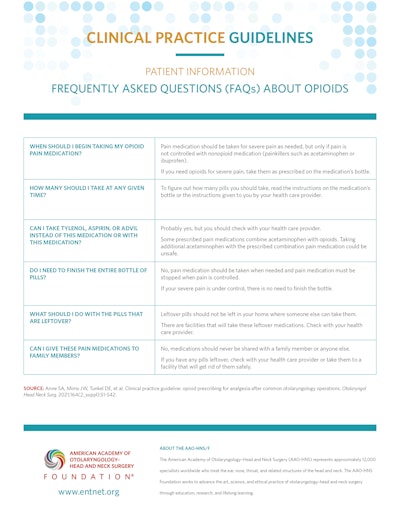
Patient Information: Frequently Asked Questions (FAQs) About Opioids
Click Here to Download PDF