2019 measures update
Quality measures are used to monitor patient care, connect clinical outcomes with healthcare processes, and meet third-party payer requirements. AAO-HNSF is committed to providing quality measures that meet our members’ requirements for public reporting to both the Centers for Medicare & Medicaid Services (CMS) and private payers, as well as to track patient care over time.
Quality measures are used to monitor patient care, connect clinical outcomes with healthcare processes, and meet third-party payer requirements. AAO-HNSF is committed to providing quality measures that meet our members’ requirements for public reporting to both the Centers for Medicare & Medicaid Services (CMS) and private payers, as well as to track patient care over time.
Current AAO-HNSF measures
The AAO-HNSF led measures development projects for Age-Related Hearing Loss, Allergic Rhinitis, Cerumen Impaction, Bell’s Palsy, Otitis Media with Effusion, Tympanostomy Tube Otorrhea, and Dysphonia. Manuscripts outlining the development process and including the measure specifications are in process and manuscripts are anticipated to be published in early 2020. These measures can be viewed on the AAO-HNSF website using: www.entnet.org/2019-measures.

Neurotology measures developed in partnership with the American Academy of Neurology have been completed and published. An executive summary is available in the October 2018 issue of Otolaryngology–Head and Neck Surgery.
CMS approval of 2019 measures
For the 2019 Qualified Clinical Data Registry (QCDR) Reg-entSM application, 25 measures were submitted to CMS and 22 were approved for use in 2019 Merit-based Incentive Payment System (MIPS) reporting. Visit www.entnet.org/2019-measures for the full list of available 2019 Reg-ent measures, measure specifications, and newly developed flows for the QCDR measures. (See page 24 in this issue for an example of a measure flow). For questions regarding measures development, please contact: measures@entnet.org.
Measure prioritization
To address future measure development, a survey of the Clinical Advisory Committee (CAC) members was conducted in two parts. The first surveyed CAC members on clinical topics derived from clinical practice guidelines (CPGs) respective to each specialty. Data results were then evaluated for measurability by AAO-HNSF staff and Richard M. Rosenfeld, MD, MPH, MBA, Senior Advisor and Methodologist for Quality Measures and Guidelines. Measure statements were developed and CAC members were asked to score the statements on the following: opportunities to improve the quality of care, perceived gap in care, and ease of data capture in an electronic health record.
The CAC leadership, with each member also representing the Reg-ent Executive Committee, include: James C. Denneny III, MD, Hearing and Balance; William R. Blythe, MD, Sinus and Allergy; Lisa E. Ishii, MD, MHS, Facial Plastics; Carol M. Lewis, MD, Head and Neck Surgery; Melissa A. Pynnonen, MD, Airway, Voice and Swallowing; Jennifer J. Shin, MD, SM, Pediatrics; Lauren S. Zaretsky, MD, General and Sleep; and Richard M. Rosenfeld, MD, MPH, MBA, Senior Advisor and Methodologist for Quality Measures and Guidelines. Each CAC Chair oversees eight to 10 specialist members. The entire CAC membership were invited to participate in the survey.
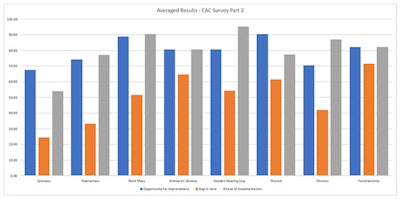
The information gathered from CAC members will inform measure needs and prioritization for the next measure development projects. (See table at left for survey results.)
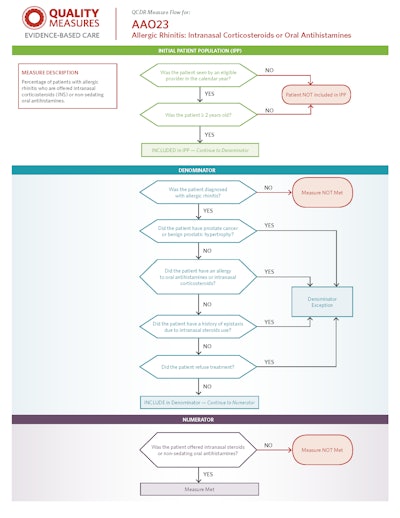 Click to Download QCDR Measure Flow AA023 PDF
Click to Download QCDR Measure Flow AA023 PDF


















