RESEARCH & QUALITY: Quality resources for your patients and practice
The Research and Quality Business Unit works to remain at the forefront of all aspects of a data-driven environment. Our daily work involves being comfortable operating within numerous highly technical areas: data analytics, decision support, clinical data registry development and operations, and clinical practice guideline and quality measure development.
The Research and Quality Business Unit works to remain at the forefront of all aspects of a data-driven environment. Our daily work involves being comfortable operating within numerous highly technical areas: data analytics, decision support, clinical data registry development and operations, and clinical practice guideline and quality measure development. Increasingly this means retaining staff who have educational and professional expertise in healthcare analytics as well as the ability to understand and work with technology in a fast-paced environment.
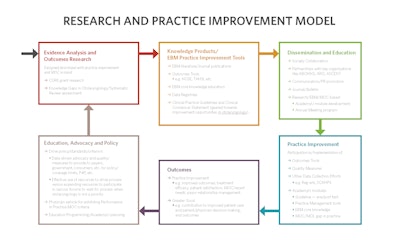 Click to Download “Research Practice Improvement Model” PDF
Click to Download “Research Practice Improvement Model” PDFIn three short years, the Reg-entSM clinical data registry has close to seven million unique patients and over 12 million patient visits. The registry analyzes millions of data points from these patients and visits and compiles the data into straightforward reports detailing benchmarks at the local and national level. The dedication of our members and their practice administrators has been incredible, and we could not have reached this milestone without their continued contributions and support.
Now we are building capabilities for research that will harness this data, ensuring it leads us toward better practices and patient outcomes in otolaryngology-head and neck care. Participating in Reg-ent will assist our members in supporting clinical investigation and pre- and post-market surveillance of devices and drugs, as well as ease the process for participating in clinical trials. For our United States-based members, Reg-ent has provided a straightforward way to report all aspects of the Merit-based Incentive Payment System (MIPS).
For close to 12 years, we have been working with our volunteer physicians on providing clinical guidance through clinical practice guidelines (CPG) and clinical consensus statements (CCS), and we are recognized by outside experts as having best practice in our development of these products. CPGs have formed the evidence base for our specialty-specific quality measures that now populate Reg-ent and are used for government (MIPS) reporting. The contribution from the Reg-ent Executive Committee (REC) and the seven Clinical Advisory Committees (CACs) to otolaryngology measurement science cannot be overstated. Staff consistently utilize the CAC’s clinical expertise to develop measures that are meaningful to our members.
This year, the Centralized Otolaryngology Research Efforts (CORE) grants has surpassed $556,562 of funding within the specialty.
A decade ago the Research and Practice Improvement Model was developed to demonstrate a future vision for Research and Quality, and it is amazing how relevant this graphic remains today as we continue to adhere to many of the aspects of this model in moving forward with research and quality priorities.



















