Upper Airway Stimulation: A New Frontier in the Treatment of OSA
Obstructive sleep apnea (OSA) affects a significant portion of the adult population. Many patients do not tolerate continuous positive airway pressure (CPAP), which is the gold-standard treatment. Over the past 30 years, numerous surgeries have been developed to enlarge the upper airway but fail to address the underlying loss of muscle tone associated with sleep onset, which is pivotal to the pathogenesis of obstructive sleep apnea.
From the Education Committees
Jeffrey J. Stanley, MD, for the General Otolaryngology and Sleep Education Committee
Obstructive sleep apnea (OSA) affects a significant portion of the adult population. Many patients do not tolerate continuous positive airway pressure (CPAP), which is the gold-standard treatment.1 Over the past 30 years, numerous surgeries have been developed to enlarge the upper airway but fail to address the underlying loss of muscle tone associated with sleep onset, which is pivotal to the pathogenesis of obstructive sleep apnea.
In contrast, upper airway stimulation (UAS), also known as hypoglossal nerve stimulation, is designed to increase upper airway muscle tone, thereby alleviating obstructive events. Stimulation of the hypoglossal nerve activates the principal upper airway dilator, the genioglossus muscle. Importantly, due to mechanical coupling of the palatoglossus muscle that insinuates itself into the intrinsic tongue musculature, there is an increase in the retropalatal airway with each anterior tongue movement. Therefore, UAS leads to an increase in both the retroglossal and retropalatal airway during sleep.
In 2014 the first upper air stimulation device, produced by Inspire Medical Systems, Inc., was approved by the Food and Drug Administration.2 In 2016 the American Academy of Otolaryngology–Head and Neck Surgery released a support statement for this mode of therapy. Indications for UAS include CPAP intolerance at age 22 years or older, body mass index (BMI) <35, apnea hypopnea index (AHI) 15-65, and absence of complete concentric collapse of the velopharynx during drug-induced sleep endoscopy.
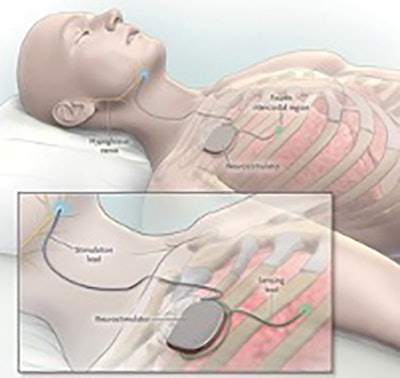 Figure 1. Hypoglossal nerve stimulator. © Inspire Medical Systems, Inc. Image used by permission.
Figure 1. Hypoglossal nerve stimulator. © Inspire Medical Systems, Inc. Image used by permission.The hypoglossal nerve stimulator consists of three components: (1) the nerve stimulator, (2) the neurogenerator, and (3) the respiratory sensor (Figure 1). During sleep, the respiratory sensor detects a change in intrathoracic pressure with inspiration leading to stimulation of the hypoglossal nerve via the neurogenerator and nerve stimulator electrode. This leads to a stiffening of the tongue as well as anterior displacement of both the tongue and soft palate.
A five-year follow-up study on patients enrolled in the initial Stimulation Therapy for Apnea Reduction (STAR) trial was published in 2018.3 The median AHI decreased from 32 to 14 at five years with good durability of effect. The response rate, defined as at least a 50 percent decrease in AHI and a postoperative AHI < 20 was 75 percent, and 44 percent had resolution of their sleep apnea with an AHI < 5. In addition, quality of life measures were significantly improved at one and five years following implantation. Nightly use was 86 percent and 80 percent at one and five years, respectively.
The ADHERE registry represents a collection of retrospective and prospective outcome measures across multiple institutions in the United States and Europe and is currently the largest cohort of patients using UAS to date. In 2008 the ADHERE registry reported strikingly similar results to those of the STAR study patients, reported above.4 Additional studies published from the registry this past year examined the potential effect of previous surgery as well as age on UAS outcomes.5,6
Serious complications related to UAS are rare, although tongue abrasion and discomfort with stimulation requiring adjustment in settings or use of a dental guard have been reported in up to 20 percent of patients.3,4 Postoperative morbidity is low with an immediate return to a normal diet and typically only mild to moderate incisional pain.
The future of UAS includes the potential expansion of current surgical indications and identification of patient factors that may affect surgical outcomes. With the known association between OSA, congestive heart failure, and arrhythmias, there is an increased likelihood of these patients needing an implantable cardiac device. Due to the proximity in the upper chest, interaction between these two devices is theoretically possible. Only a small case series of patients with both a cardiac implant and an UAS implant have been reported with no device-to-device interactions observed, but further research is needed to address potential safety concerns.7 Finally, current indications for UAS include adult-aged patients only. A recent study on nonobese patients with Down syndrome, ages 10-21 years, reported promising results for this patient population, who often have a combination of generalized hypotonia and structural abnormalities accounting for their high prevalence of OSA and high-failure rate following adenotonsillectomy.8
In summary, the overall efficacy of UAS is high, with approximately 70 percent responding to therapy, and surgical morbidity is low in comparison to traditional structural surgery for OSA. There is growing enthusiasm for this surgical procedure, since for the first time in many years we are now seeing high numbers of patients (> 40 percent) with resolution of their OSA with UAS therapy. As with any relatively new procedure, many questions remain, including ideal patient selection, long-term compliance rates, durability of results, the effect of fluctuations in BMI, the effect of prior or additional structural surgeries and the long-term effect on cardiovascular morbidity and mortality associated with OSA. Research on all these topics is underway as UAS appears to be a new frontier in the surgical treatment of OSA.
References
- Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147(4):887-95.
- Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014 Jan;370(2):139-49.
- Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194-202.
- Boon M, Huntley C, Steffen A, et al. Upper airway stimulation for obstructive sleep apnea: results from the ADHERE registry. Otolaryngol Head Neck Surg. 2018;159(2):379-385.
- Huntley C, Vasconcellos A, Doghramji K, et al. Upper airway stimulation in patients who have undergone unsuccessful prior palate surgery: an initial evaluation. Otolaryngol Head Neck Surg. 2018;159(5):938-940.
- Withrow K, Evans S, Harwick J, et al. Upper airway stimulation response in older adults with moderate to severe obstructive sleep apnea. Otolaryngol Head Neck Surg. 2019;161(4):714-719.
- Parikh V, Thaler E, Kato M, et al. Early feasibility of hypoglossal nerve upper airway stimulator in patients with cardiac implantable electronic devices and continuous positive airway pressure-intolerant severe obstructive sleep apnea. Heart Rhythm. 2018;15(8):1165-1170
- Diercks GR, Wentland C, Keamy D, et al. Hypoglossal nerve stimulation in adolescents with down syndrome and obstructive sleep apnea. Otolaryngol Head Neck Surg. 2018;144(1):37-42.