Data and evidence
One of the most frustrating subjects permeating the medical community revolves around data and evidence. There has been a steady progression, particularly over the last decade, of the realization and acceptance that data and evidence should be the foundation of the diagnosis and treatment of medical problems.
 James C. Denneny III, MD
James C. Denneny III, MD
AAO-HNS/F EVP/CEO
Even though the pace of progress has not satisfied everybody, there has been clear advancement in the volume and quality of available evidence. Data scientists are constantly working to define exactly what constitutes the best and most valuable data elements needed to address the problem at hand. This transition phase of identifying what data has value and what is little more than busywork has created a catch-22 situation for physicians who genuinely want to define and provide the most effective care possible.
Perhaps the greatest single cause of burnout and the decreasing wellness of the physician community emanates from this issue. We are all aware of the widespread dissatisfaction with the current EHR documentation requirements and adaptation to a satisfactory clinical flow. We are also aware that most of the data captured does not serve to improve patient care or measure elements important to the patients themselves. The perception that MIPS reporting also does little to advance patient care, but is required to avoid penalties in payment for work already performed, does little to engender physician support of the process. When you add in the heterogeneous interpretation of available clinical data, particularly diagnostic and therapeutic modalities by the insurance industry, you have a formula for exasperation and anger. It is difficult to explain to physicians that they are expected to foot the cost, both monetary and temporal, for collecting data that in many cases is superfluous, and that they can be controlled by policies based on minimal, if any, acceptable evidence.
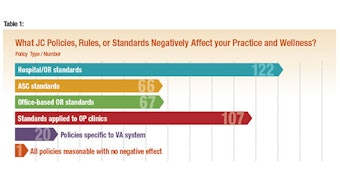
An area that is particularly infuriating to many otolaryngologists is based on policies and standards propagated by hospital systems and the Joint Commission that are used in the hospital and ambulatory surgery setting and have now expanded into office practice. Increasing discontent among our members over the last several years seemed to explode recently on ENTConnect. A multiquestion survey was disseminated both on ENTConnect and through OTO News.
Over the two weeks the survey was open, we received a substantial response from 158 members, representing academic, hospital system, military, and private practice settings. Most of these participants took the time to add personal comments. The general concerns included staff and physician frustration, wasted time, increased costs, and patient safety and quality issues. Specific concerns included the requirement for “peel-packing” office instruments, general sterilization procedures, specific sterilization policies for flexible endoscopes, suction canister and tubing rules, and single vial medicine usage. Details of the survey are presented on pages 6-7.
The American College of Surgeons had previously conducted a study on the “surgical caps” issue and demonstrated that recommended standards using the bouffant hat were the most likely to cause infection. Even with that data, many ORs have not rescinded the policy. We plan on pursuing direct discussions with the Joint Commission and hope to use the results of this study to trigger a review of some of these policies. AMA Trustee Russell W.H. Kridel, MD, an otolaryngologist and facial plastic surgeon from Houston, TX, has volunteered to help enlist the AMA in this discussion, which could result in creation of a system that works for everybody.
The FDA held its first workshop on devices for sleep disordered breathing on April 16, 2018. The Academy was closely involved in the planning of this watershed conference, which included seven organizations that care for these patients. The workshop concentrated on discussing and defining measurement criteria that would be applicable in future studies for sleep-related treatment modalities. These included definitions of apnea, hypopnea, obstructive sleep apnea, and central sleep apnea. There was a robust discussion about what should be measured, both in terms of diagnostic and therapeutic modalities. Finally, there was extensive discussion on emerging digital technology and future prescription and OTC products and how they should be regulated.
I want to thank the team that represented the AAO-HNS—led by Kathleen Yaremchuk, MD, MSA, and including Raj Dedhia, MD; M. Boyd Gillespie, MD, MSc; Stacey L. Ishman, MD, MPH; Ofer Jacobowitz, MD, PhD; Edward M. Weaver, MD, MPH; and myself—for the fantastic contributions made.