Role of Panel Members in the Development and Dissemination of Quality Products
The roles and responsibilities of relevant stakeholders is explained.
The American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) is committed to the production, dissemination, and implementation of quality-driven, evidence-based Clinical Practice Guidelines (CPGs) and Expert Consensus Statements (ECSs). Since 2006, the AAO-HNSF has developed quality products to provide statements or recommendations for action based on best evidence using methodologic standards defined in the CPG and Consensus Statement development manuals.
To ensure the validity and applicability of quality products, a panel is convened from both external and internal AAO-HNS/F organizations. This includes Academy members from AAO-HNS/F committees and sections as well as external representatives from consumer and patient groups, partner organizations, and other otolaryngology societies. For CPGs, the panel includes multidisciplinary participation with relevant stakeholders, which helps identify and evaluate all relevant evidence, builds support among the intended guideline users, and increases the chances of addressing practical problems related to implementation.
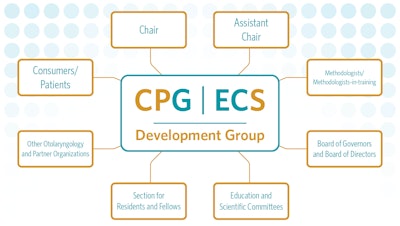
Panel members can serve in one of many roles in the development group. The chair serves as the leader responsible for ensuring that methodologic standards are followed and overseeing the interpersonal dynamics within the group. The assistant chair supports the chair in their responsibilities and is chosen based on their expertise in the content area and evidence-based medicine. They may also be asked to chair future quality products.
Methodologists are assigned to the working group to ensure adherence to methodologic standards and protocols. They provide support to the chair during conference calls and meetings to help facilitate the prescribed processes for guideline development. Methodologists-in-training are included to gain the necessary skills and knowledge to serve as methodologists for future guideline groups.
Academy members are invited to the development group to serve in various roles and responsibilities. Representatives from the Board of Governors and Board of Directors are invited to facilitate dissemination of quality products and ensure that the input represents the needs of AAO-HNS members. Relevant Education and Scientific AAO-HNSF Committees are asked to nominate a member to assist with implementation and align Academy education products with quality products. A member from the Section for Residents and Fellows-in-Training (SRF) is included on these panels to help meld the quality products with resident and fellow education curricula.
Other otolaryngology societies, partner organizations, and consumer/patient groups (Figure 1) are also invited to participate in the development group as stakeholders on relevant topics. Guideline panels often include primary care clinicians, nurses, physician assistants, pediatricians, geriatricians, audiologists, as well as other specialties and otolaryngology societies. A consumer/patient representative is invited to join guideline development groups to provide a layperson perspective and to advocate for the patient who is the focus of the guideline.
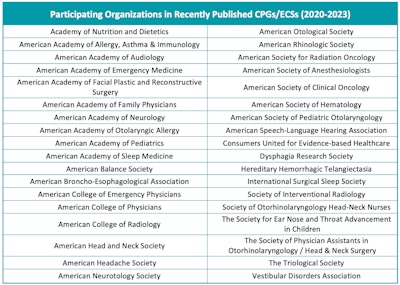 Figure 1. List of recent external organizations that participated in the development of CPG/ECS.
Figure 1. List of recent external organizations that participated in the development of CPG/ECS.
Dissemination of Quality Products
The AAO-HNSF provides a number of resources to raise awareness and promote the dissemination of published CPGs and ECSs. Dissemination plays a crucial role in ensuring that quality products reach all relevant stakeholders. Through effective dissemination, stakeholders can gain access to the latest evidence-based statements and recommendations to identify quality improvement opportunities.
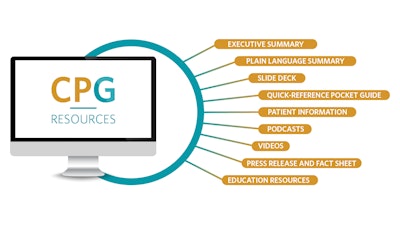
Each CPG includes supplemental resources such as an executive and plain language summary, patient handouts, pocket guides, slide decks, videos, and fact sheets. CPGs are also used to develop patient information on ENTHealth.org. In addition, both CPGs and ECSs are featured on the Otolaryngology–Head and Neck Surgery journal podcast series and as an education resource on OTO Logic. A list of all published CPGs and ECSs and supplemental resources can be found on the AAO-HNSF CPG Webpage and ECS Webpage.
2023 Published Expert Consensus Statements
Dysphagia in Head and Neck Cancer Patients
ECS on the management of dysphagia in head and neck cancer (HNC) patients to address controversies and offer opportunities for quality improvement.
Pediatric Persistent Obstructive Sleep Apnea After Adenotonsillectomy
ECS regarding persistent pediatric obstructive sleep apnea (OSA) focused on quality improvement and clarification of controversies.
Resources
Rosenfeld RM, Nnacheta LC, Corrigan MD. Clinical consensus statement development manual. Otolaryngol Head Neck Surg. 2015; 153(2 suppl): S1- S14.
Rosenfeld RM, Shiffman RN, Robertson P. Clinical practice guideline development manual, third edition: a quality-driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013; 148(1): S1– S55.