Highlights of Research and Quality Initiatives
The resources needed to navigate the ever-changing landscape of research, quality, and performance measurement.
Vikas Mehta, MD, MPH, AAO-HNSF Coordinator of Research and Quality

The Research and Quality Business Unit is comprised of the following key Foundation initiatives:
- Reg-entSM Clinical Data Registry
- Quality Measures Development
- Clinical Practices Guidelines and Expert Consensus Statements (Development, Implementation and Dissemination)
- Centralized Otolaryngology Research Efforts (CORE) grants program
In addition, the work of the Outcomes Research and Evidence-based Medicine (OREBM) and Patient Safety Quality Improvement (PSQI) Committees are supported by the staff in Research and Quality.
The Research and Quality Business Unit staff in conjunction with our dedicated member volunteers have been diligently focused on developing products and tools to assist members navigate the ever-changing landscape of research, quality, and performance measurement. Our goal is to have the tools, information, and processes in place for our members to successfully participate with these endeavors and incorporate the best evidence and quality improvement measures into their practice.
We are in the seventh year of operating the Reg-ent clinical data registry. We continue to meet the rigorous requirements set by the Centers for Medicare & Medicaid Services (CMS) to operate as a Qualified Clinical Data Registry (QCDR) and support our members and their practices in reporting to the Merit-based Incentive Payment System (MIPS). Our member practices are working closely with Reg-ent and our registry partner, MRO (formerly FIGmd), to ensure data is captured and mapped appropriately.
However, Reg-ent is much more than a reporting tool. This past year we have finalized Reg-ent research policies and procedures and formalized the Reg-ent Research Advisory Group (RRAG) to prioritize and approve research studies utilizing the Reg-ent data repository. Working with OM1, we now have a robust data repository of research-grade data that will allow for approved studies to proceed. This data will also allow us to collect pre- and post-market data on drugs and devices, provide support for Academy advocacy efforts, allow more real-world investigation of outcomes using data from both the private and academic sectors, and continue to prepare and assist members with meeting requirements for government and private payer quality initiatives. The more members we have contributing, the more robust and inclusive the data will be, and so we look forward to having a majority of otolaryngologist-head and neck surgeons participate in Reg-ent.
A key aspect of Reg-ent development is to continue to have meaningful quality measures. Many of the current and future measures have benefited from a robust clinical practice guideline process and the continued development of guidelines on important topics across the specialty. This year, we will put forward several new quality measure topics to CMS (dysphonia, neck mass, and thyroidectomy nerve injury). Two Expert Consensus Statements (ECS) were published in 2023: Dysphagia in Head and Neck Cancer Patients and Pediatric Persistent Obstructive Sleep Apnea After Adenotonsillectomy.
Members and staff continue to monitor the national quality landscape organizations. Staff participate in the American College of Surgeons Surgical Quality Alliance (SQA). In addition, we keep up to date on new and updated regulations impacting clinical data registries through our participation with the Physician Clinical Registry Coalition (PCRC). We are also working on developing quality metrics that may be more relevant to medical centers and practices that are performing higher acuity, tertiary otolaryngology care and hope to be able to incorporate and offer those next year.
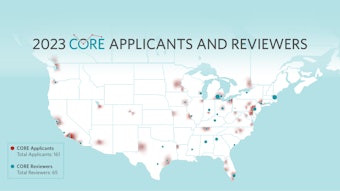
This issue of the Bulletin also outlines our 2023 Centralized Otolaryngology Research Efforts (CORE) awardees. Please take time to review the list of those achieving awards and the list of grants that were conferred through the virtual CORE Study Section that was held in March. We congratulate all the recipients!