The Current State of Implantable Hearing Devices and MRI Compatibility
Despite advances to improve MRI compatibility, barriers still exist for patients with cochlear, bone conduction and auditory brainstem implants.
Kristan P. Alfonso, MD, Jason A. Brant, MD, Anita Jeyakumar, MD, MS, and J. Thomas Roland, Jr., MD, on behalf of the Implantable Hearing Devices Committee
IMDs are broadly classified into two categories: passive IMDs (PIMDs), which do not include any electronic components, and active IMDs (AIMDs), which do. Examples of PIMDs include plates and screws that are used to fix broken bones, dental implants, and coronary stents; examples of AIMDs include pacemakers, deep brain stimulators, and implantable hearing devices.
MRI relies on strong magnetic fields to create images of the inside of the body. Although any implant could be impacted by these magnetic fields, there are specific characteristics of each that make them more or less likely to be affected or that might lead to complications. Some of these complications include movement of the IMD, resulting in displacement and pain, or heating due to electrical currents created by moving within the magnetic field. AIMDs have additional considerations owing to their electronics, which can malfunction or become damaged after being exposed to the magnet.3 Not only can the IMD be affected by the scan, but the presence of the device can impact the quality of the image that is obtained. The metal components present in the device can create distortions (shadows, so that the tissue adjacent to the device is not seen on the MRI), making some or all parts of the MRI scan difficult to evaluate properly. Magnets used in hearing implants, for example, can have a more significant impact on the image. In some cases, an IMD must be partially or completely removed to complete a diagnostic scan. Below, we explore the interaction between cochlear and bone conduction hearing devices and MRI, examining both how these implants affect MRI procedures and how MRI can influence the function and integrity of the implants.
MRI in patients with CIs, bone conduction hearing devices, and auditory brainstem implants (ABIs) present numerous challenges because of the ferromagnetic component of the system. In general, an implant is defined as “MRI unsafe” if it is absolutely contraindicated to have an MRI, “MRI safe” if an MRI is possible under all conditions, and “MRI conditional” if certain conditions must be met to have an MRI.4 Most implantable hearing devices are MRI conditional. Conditions can be met if the magnet is removed, a headwrap is placed, or a lower Tesla machine is used.
Previously, CIs were an absolute contraindication (MRI unsafe) because of the presence of an internal magnet in the former. Although helpful to optimize signal transmission between the external auditory and internal processing components of the CIs, the magnets can be problematic during MRIs, interfering with the machine’s magnetic field. Interference between the MRI and magnet can lead to displacement of metallic objects, induction of heat and currents, demagnetization, reversal of magnetic polarity, or image degradation.5
To initially address this, CI designs evolved to enable MRI imaging by binding the CI with a headband and designing surgically removable and replaceable internal magnets. MED-EL implants (Sonata, Pulsar, Concert, and Concert Pin; MED-EL GmbH) are approved for use with MRI instruments involving field strengths up to 1.5 T if head dressings are used. Headwraps should be performed with tight dressings to counteract any torque or translational forces. FDA-approved headwrap kits (Cochlear Ltd) have been developed. CIs with removable magnets, such as the Nucleus Freedom, Nucleus 24, certain Nucleus 22 models (Cochlear), and Clarion HiRes 90K (Advanced Bionics AG) are approved for use with MRI systems up to 1.5 T following removal of the internal magnet.6 However, these approaches have various adverse effects including pain, magnet damage, displacement of the magnet or polarity reversal of the magnet, and increased risk of infection.7 These complications sometimes need surgical intervention to replace the magnet or to explant and then reimplant the device.
Cases of weakening internal magnet strength due to demagnetization have been reported with previous generations of CIs. Weakening or demagnetization makes it necessary to use a stronger external magnet or may nece.7
The magnetic component of CIs causes artifacts on MRI, primarily MRI of the brain and head and neck. Post-implantation MRI can lead to artifacts generated by either the magnet (if left in place) or the electrode (which contains titanium).7 The presence of an MRI artifact of any size can negatively influence the diagnostic accuracy of imaging studies in patients with CIs. Moreover, larger artifacts may further obscure regions of interest, potentially leading to challenges in identifying pathologies or assessing treatment efficacy.8 A 2015 study reported the mean (SD) artifact size was 7.43 cm (2.03 in) (range, 4.55–9.87 cm) along the long axis and 4.16 cm (1.19 in) (range, 3.00–6.80 cm) along the short axis, with the largest artifacts noted in bilaterally implanted patients. There appeared to be a decreased artifact size with 1.0-T MRI, but this is not commonly used.9 Two studies reported that in a 1.5-T MRI, the artifact is 6.6-7.0-cm long in the anterior-posterior dimension and 4.8 cm (1.89 in) in the lateral dimension, centered around the device.10,11 A cadaveric study in implanted cadavers with the 3.0-T MRI demonstrated that the image quality of the nearby brain regions and some of the contralateral hemisphere were limited.12
A 2019 study of MRI-related adverse events reported to the FDA reviewed 1,568 events from January 2008 to December 2017.13 Thermal events were most frequently reported (59%), of which 39% had an unclear etiology. MRI conditional devices can heat and lead to patient injury under other conditions.13 According to a review by Dempsey and Condon, “Although detailed studies concerning the burn hazard in MRI have not been reported, it is widely believed that direct electromagnetic induction in looped cables associated with the patient is responsible for the excessive heating.”14
In lieu of the headbands, scientists developed freely rotatable diametric magnets, which allowed the CI magnets to align with the external magnetic field created by the MRIs aiming to minimize pain and discomfort for patients.5,15,16 Implants from the three major manufacturers have one to four rotating magnets that result in less torque, reducing the incidence of pain, even during a high-induction MRI. The implants with this technology include the MedEl Synchrony (MedEl), HiRes Ultra3D (Advanced Bionics), and Cochlear Nucleus Profile Plus (Cochlear Corp).
Regarding bone conduction hearing devices, traditional percutaneous devices (Cochlear Connect and Oticon Ponto) as well as passive percutaneous devices (Cochlear Attract and Medtronic Sophono) are MRI conditional. The newer active transduction transcutaneous devices (MedEl Bonebridge and Cochlear Osia) are also MRI conditional. The Cochlear Osia OSI200 requires an FDA-approved headwrap or magnet removal, whereas the Cochlear Osia OSI300 is MRI conditional without headwrap or magnet removal.
Regarding ABIs, the MedEl ABI has an MRI-compatible magnet whereas the cochlear ABI does not. The cochlear ABI is the only device approved for use in the U.S. For cochlear ABIs, the magnets are removed for NF2 patients at implantation, and the patients require use of an external retention disc. A dilemma occurs regarding whether to implant with or without a magnet for other children receiving an ABI—for which the main indications include no cochleae or cochlear nerves, severe anomalies of the inner ear with cochlear nerve deficiency, and probable poor CI outcome.
Recent work has explored methods for artifact reduction by altering MRI sequences. Image quality depends on the location of the internal magnet, the angle of the external MRI magnet to the internal magnet, and the protocols of the MRI sequences being performed. Sharon et al. compared 14 MRI sequences in patients with unilateral CI and the effect on IAC and CPA visibility. Recommendations include thin slices, use of CISS and FIESTA sequences with images in all three anatomic planes. They note reduced visibility in T2 and FLAIR sequences and recommend against fat saturation and DWI sequences.17
Despite advances to improve compatibility between implantable hearing devices and MRI scanning, many existing implant recipients have older technology with more limitations. It is important for the radiologist and otolaryngologist to be aware of the implant type and how a scan may safely be performed to generate a diagnostic-quality image. Implant companies should develop magnets that are MRI conditional up to 3.0 T without the need for headwrap or magnet removal and develop processes to help patients with older technology adapt to the current state of MRI.
Table 1. Overview of Magnetic Resonance Imaging (MRI) Safety Guidelines for Commonly Used Cochlear and Osseointegrated Implant Devices.
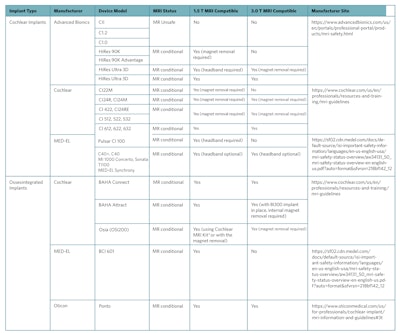
Reproduced from Abdalla, I., Choi, J.S., Struyk, G., Adams, M.E. and Huang, T.C. (2024), Magnetic Resonancy Imaging Safety in Active Osseointegrated (Osia®) and Cochlear Implants: New Technology Creating Confusion. The Laryngoscope, 134: 393-396. https://doi.org/10.1002/lary.30808
* The MRI Kit includes round magnetic splints (to be placed on the skin over each implant magnet site), chinstraps and bandage to provide compression on each splint at the implant magnet site. Manufacturer user guidelines are available at https://www.cochlear.com/us/en/professionals/resources-and-training/mri-guidelines.
References
- Kuroda K, Yatsushiro S. New Insights into MR Safety for Implantable Medical Devices. Magn Reson Med Sci. 2022 Mar 1;21(1):110-131.
- Gopinathannair R, Mar PL, Gandhi G, et al. Incidence and predictors of MRI scan utilization in MRI-conditional pacemaker recipients: A multicenter experience. Pacing Clin Electrophysiol. 2018 Nov;41(11):1519-1525.
- Aissani S, Laistler E, Felblinger J. MR safety assessment of active implantable medical devices. Radiologe. 2019 Dec;59(Suppl 1):40-45.
- Abdalla I, Choi JS, Struyk G, Adams ME, Huang TC. Magnetic Resonancy Imaging Safety in Active Osseointegrated (Osia®) and Cochlear Implants: New Technology Creating Confusion. Laryngoscope. 2024 Jan;134(1):393-396.
- Pietro Canzi, Aprile F, Simoncelli A, et al. MRI-induced artifact by a cochlear implant with a novel magnet system: an experimental cadaver study. Eur Arch Otorhinolaryngol. 2020;278(10):3753-3762.
- Kim BG, Kim JW, Park JJ, Kim SH, Kim HN, Choi JY. Adverse Events and Discomfort During Magnetic Resonance Imaging in Cochlear Implant Recipients. JAMA Otolaryngol Head Neck Surg. 2015;141(1):45–52.
- Bawazeer N, Vuong H, Riehm S, Veillon F, Charpiot A. Magnetic resonance imaging after cochlear implants. J Otol. 2019 Mar;14(1):22-25.
- Kalmanson OA, Talmage GD, Honce JM, Gubbels SP. Effect of Head Position and Magnetic Resonance Sequence on Cochlear Implant–Related Artifact Size and Internal Auditory Canal Visibility. Otology & Neurotology. 2023;44(2):e73-e80.
- Cass ND, Honce JM, O’Dell AL, Gubbels SP. First MRI With New Cochlear Implant With Rotatable Internal Magnet System and Proposal for Standardization of Reporting Magnet-Related Artifact Size. Otology & Neurotology. 2019;40(7):883-891.
- Todt I, Guerkov R, Gehl HB, Sudhoff H. Comparison of Cochlear Implant Magnets and Their MRI Artifact Size. BioMed Research International. 2020;2020:e5086291.
- Baumgartner WD, Youssefzadeh S, Hamzavi J, Czerny C, Gstoettner W. Clinical application of magnetic resonance imaging in 30 cochlear implant patients. Otol Neurotol. 2001;22(6):818-822.
- Vincent C, Ruzza I, Vaneecloo FM, Dubrulle F. Magnetic resonance imaging with the Digisonic SP Neurelec cochlear implant. Eur Arch Otorhinolaryngol. 2008;265(9):1043-1046.
- Delfino JG, Krainak DM, Flesher SA, Miller DL. MRI‐related FDA adverse event reports: A 10‐yr review. Medical Physics. 2019 Dec;46(12):5562-71.
- Dempsey, Mary F., and Barrie Condon. “Thermal injuries associated with MRI.” Clinical Radiology 56, no. 6 (2001): 457-465.
- Cass ND, Totten DJ, Ross JD, O’Malley M. Characterizing Cochlear Implant Magnet-Related MRI Artifact. Annals of Otology, Rhinology & Laryngology. 2022;132(3):250-258.
- Majdani O, Rau TS, Götz F, et al. Artifacts caused by cochlear implants with non-removable magnets in 3T MRI: phantom and cadaveric studies. Eur Arch Otorhinolaryngol. 2009;266(12):1885-1890.
- Sharon JD, Northcutt BG, Aygun N, Francis HW. Magnetic Resonance Imaging at 1.5 Tesla With a Cochlear Implant Magnet in Place: Image Quality and Usability. Otol Neurotol. 2016 Oct;37(9):1284-90.